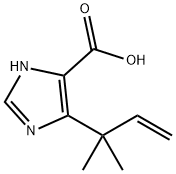
1H-Imidazole-5-carboxylic acid, 4-(1,1-dimethyl-2-propen-1-yl)- synthesis
- Product Name:1H-Imidazole-5-carboxylic acid, 4-(1,1-dimethyl-2-propen-1-yl)-
- CAS Number:714273-87-7
- Molecular formula:C9H12N2O2
- Molecular Weight:180.2

60100-09-6
0 suppliers
inquiry

316148-57-9
1 suppliers
inquiry

714273-87-7
3 suppliers
inquiry
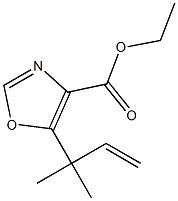
714273-86-6
0 suppliers
inquiry
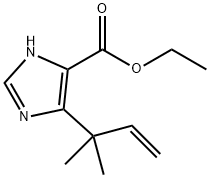
316148-58-0
2 suppliers
inquiry
Yield:714273-86-6 7% ,316148-58-0 36% ,714273-87-7 9%
Reaction Conditions:
in water at 150; for 5 h;
Steps:
3.h 5-(1,1-Dimethyl-allyl)-3H-imidazole-4-carboxylic acid ethyl ester
A suspension of 2-chloro-4,4-dimethyl-3-oxo-hex-5-enoic acid ethyl ester (19.4 g, 0.09 mol) and water (1.94 ml, 0.11 mol) in formamide (36.8 ml) was shaken briefly, then dispensed into 15×18 ml vials. The vials were sealed and heated at 150° for 5 h. After cooling to room temperature, the vials' contents were combined and extracted exhaustively with chloroform. The extracts were dried and evaporated to afford a concentrated formamide solution (14.7 g). This was added to a silica column (7 cm diameter, 11 cm height) packed in 1% MeOH/1% Et3N in chloroform. Elution of the column with 2 L of this mixture followed by 2 L of 2% MeOH/1% Et3N in chloroform afforded, in the early fractions, a compound suspected of being 5-(1,1-dimethyl-allyl)-oxazole-4-carboxylic acid ethyl ester (1.23 g. 7%).HPLC (214 nm) tR=8.68 (50.4%) min.1H NMR (400 MHz, CDCl3) δ 1.40 (t, J=7.2 Hz, 3H, -CH2CH3); 1.54 (s, 6H); 4.38 (t, J=7.2 Hz, 2H, -CH2CH3); 5.03 (d, J=17.4 Hz, 1H, -CHCH2); 5.02 (d, J=10.4 Hz, 1H, -CHCH2); 6.26 (dd, J=17.4, 10.4 Hz, 1H, -CHCH2); 7.83 (s, 1H).LCMS tR=8.00 (210.1 [M+H]+, 361.1 [2M+H]+) min.Recovered from later fractions was the desired 5-(1,1-dimethyl-allyl)-3H-imidazole-4-carboxylic acid ethyl ester (3.13 g, 17%). (Hayashi et al., J. Org. Chem., 65:8402-8405 (2000)).HPLC (214 nm) tR=5.52 (96.0%) min.1H NMR (400 MHz, CDCl3) δ 1.38 (t, J=7.0 Hz, 3H); 1.57 (s, 6H); 4.35 (q, J=7.0 Hz, 2H); 5.04-5.14 (m, 2H, -CHCH2); 6.28 (dd, J=18.0, 10.4 Hz, 1H, -CHCH2); 7.52 (s, 1H).LC/MS tR=5.30 (209.1 [M+H]+, 417.2 [2M+H]+) min.Additional 5-(1,1-dimethyl-allyl)-3H-imidazole-4-carboxylic acid ethyl ester was also recovered from the column (3.59 g, 19%) which was of lower purity but still sufficient for further reaction.Another byproduct isolated from a similar reaction (smaller scale) by further elution of the column with 5% MeOH/1% Et3N in chloroform was a compound suspected of being 5-(1,1-dimethyl-allyl)-3H-imidazole-4-carboxylic acid (0.27 g, 9%).HPLC (245 nm) tR=5.14 (68.9%) min.1H NMR (400 MHz, CD3OD) δ 1.45 (s, 6H); 4.97 (d, J=10.6 Hz, 1H, -CHCH2); 5.01 (d, J=17.7 Hz, 1H, -CHCH2); 6.28 (dd, J=17.7, 10.6 Hz, 1H, -CHCH2); 7.68 (s, 1H).LCMS tR=4.72 (181.0 [M+H]+, 361.1 [2M+H]+) min.
References:
US2008/221122,2008,A1 Location in patent:Page/Page column 22-23