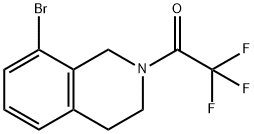
1-(8-BroMo-3,4-dihydroisoquinolin-2(1H)-yl)-2,2,2-trifluoroethanone synthesis
- Product Name:1-(8-BroMo-3,4-dihydroisoquinolin-2(1H)-yl)-2,2,2-trifluoroethanone
- CAS Number:726136-49-8
- Molecular formula:C11H9BrF3NO
- Molecular Weight:308.09

50-00-0
846 suppliers
$10.00/25g
![N-[2-(3-BROMO-PHENYL)-ETHYL]-2,2,2-TRIFLUORO-ACETAMIDE](/CAS/GIF/215797-81-2.gif)
215797-81-2
12 suppliers
inquiry

252331-63-8
18 suppliers
$188.00/1g

726136-49-8
8 suppliers
inquiry
Yield:252331-63-8 33% ,726136-49-8 36%
Reaction Conditions:
with sulfuric acid in acetic acid at 20; for 72 h;
Steps:
288
A mixture of acetic acid (61 mL) and concentrated H2SO4 (40 mL) was added to a mixture of intermediate I-61 (11 g, 37 mmol, 1.0 eq) and paraformaldehyde (1.8 g, 59 mmol, 1.6 eq) and the reaction mixture was stirred at room temperature for 72 hours. The crude reaction mixture was poured onto a mixture of ice and water and extracted with ethyl acetate. The combined organic layer was washed with a saturated aqueous solution of NaHCO3 and water and the combined organic layers were dried over anhydrous Na2SO4, the solids were removed by filtration and the filtrate was concentrated by evaporation. The crude reaction product was purified by silica gel column chromatography to give pure intermediate I-62 (3.8 g, 33%) and a mixture of intermediates I-62 and I-62' (4.0 g, 36%) that was discarded. 1H-NMR (400 MHz, CDCl3) δ: 7.337.37 (m, 2H), 7.00 (d, 1H, J=8.4 Hz), 7.02 (d, 1H, J=8.4 Hz), 4.72 (m, 2H), 3.84 (m, 2H), 2.912.96 (m, 2H). MS (ESI): m/z 308 (M+H+).
References:
US2010/204214,2010,A1 Location in patent:Page/Page column 110-111
![N-[2-(3-BROMO-PHENYL)-ETHYL]-2,2,2-TRIFLUORO-ACETAMIDE](/CAS/GIF/215797-81-2.gif)
215797-81-2
12 suppliers
inquiry

64-19-7
1537 suppliers
$10.00/25ML

252331-63-8
18 suppliers
$188.00/1g

726136-49-8
8 suppliers
inquiry