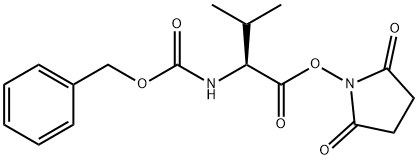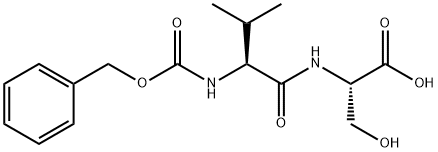
(2S)-2-[(2S)-2-{[(benzyloxy)carbonyl]amino}-3-methylbutanamido]-3-hydroxypropanoic acid synthesis
- Product Name:(2S)-2-[(2S)-2-{[(benzyloxy)carbonyl]amino}-3-methylbutanamido]-3-hydroxypropanoic acid
- CAS Number:72635-81-5
- Molecular formula:C16H22N2O6
- Molecular Weight:338.36
Yield:72635-81-5 138 mg
Reaction Conditions:
with sodium hydrogencarbonate in tetrahydrofuran;water at 0; for 0.5 h;Green chemistry;
Steps:
A Typical Procedure of the Amidation of Cbz-L-Phenylalanine (6a) Using Ethyl Chloroformate is as Follows.
General procedure: To acolorless solution of 150 mg (0.50 mmol) of Cbz-L-phenylalanine (6a) in 10 mL of THF were added at 0 °C 67 L (0.70mmol, 1.4 equiv) of ethyl chloroformate and 209 L (1.5 mmol,3.0 equiv) of triethylamine. After stirring for 30 min at 0 °C, asolution of 110 mg (0.75 mmol, 1.5 equiv) of L-Glu-OH (2e)and 63 mg (0.75 mmol, 1.5 equiv) of NaHCO3 in 10 mL of H2Owas added at 0 °C to the colorless suspension. The mixture wasstirred for 30 min at 0 °C and the resulting colorless clear solution was concentrated in vacuo. The residue was adjusted topH 2 by addition of a 1.0 M aqueous solution of HCl. Thecolorless suspension was diluted with 10 mL of brine, extractedwith 25 ml 3 of a 4:1 mixture of EtOAc and MeOH, anddried over anhydrous MgSO4. The crude product was chromatographed on silica gel with a 9:1 mixture of chloroform andMeOH containing 1% AcOH to afford 187 mg (87% yield) ofCbz-L-Phe-L-Glu-OH (7ae).
References:
Ezawa, Tetsuya;Jung, Seunghee;Kawashima, Yuya;Noguchi, Takuya;Imai, Nobuyuki [Bulletin of the Chemical Society of Japan,2017,vol. 90,# 6,p. 689 - 696]
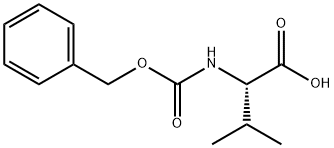
1149-26-4
541 suppliers
$5.00/1g
![(2S)-2-[(2S)-2-{[(benzyloxy)carbonyl]amino}-3-methylbutanamido]-3-hydroxypropanoic acid](/CAS/20200119/GIF/72635-81-5.gif)
72635-81-5
12 suppliers
inquiry
![L-Valine, N-[(phenylmethoxy)carbonyl]-, anhydride with ethyl hydrogen carbonate](/CAS/20211123/GIF/119153-86-5.gif)