
4-fluoro-5-oxo-2,4-bis(trifluoromethyl)-1,3-dioxolane-2-carbonyl fluoride synthesis
- Product Name:4-fluoro-5-oxo-2,4-bis(trifluoromethyl)-1,3-dioxolane-2-carbonyl fluoride
- CAS Number:7345-49-5
- Molecular formula:C6F8O4
- Molecular Weight:288.05
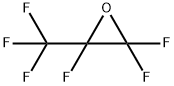
428-59-1
208 suppliers
inquiry

7345-49-5
14 suppliers
$95.00/1 g
Yield: 86%
Reaction Conditions:
with 4-methoxy-benzaldehyde at 85 - 140; under 1500.15 - 5250.53 Torr; for 19 h;Autoclave;Large scale;Temperature;Reagent/catalyst;Pressure;
Steps:
1; 2; 3; 4; 5; 6; 7; 8; 9; 10; 11; 12; 13; 14; 15; 16; 17; 1 Example 15 Preparation of trifluoropyruvic acid fluoride dimer
Using the same reactor as in Example 1, 4-methoxybenzaldehyde (2.38 kg, 17.48 mol) was charged and heated to 85 ° C., while maintaining the same temperature, hexafluoropropylene oxide (3.15 kg, 18.1 mol). 97 mol) was continuously supplied over 7 hours to carry out the reaction step.The pressure during this period was a maximum of 0.2 MPa. Next, the reaction was performed at a reaction temperature of 140 ° C. for 12 hours. The maximum pressure at 140 ° C. was 0.7 MPa.After completion of the reaction, the mixture was cooled to room temperature and then separated to obtain a crude trifluoropyruvic acid dimer (light yellow transparent liquid, 2.35 kg).In the quantification by 19F-NMR using benzotrifluoride as an internal standard,2.17 kg (7.53 mol) of the desired product, trifluoropyruvic acid dimer, was produced (86% yield, based on 4-methoxybenzaldehyde).
References:
Tosoh Corporation;Tosoh Finechem Corporation;Miyauchi, Hideki;Matsuo, Hiroshi;Kagawa, Takumi;Kondo, Norihisa JP2020/37548, 2020, A Location in patent:Paragraph 0027-0050; 0052
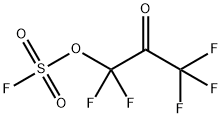
51689-98-6
0 suppliers
inquiry

7345-49-5
14 suppliers
$95.00/1 g

428-59-1
208 suppliers
inquiry

51689-98-6
0 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

7309-84-4
14 suppliers
$45.00/1g

7345-49-5
14 suppliers
$95.00/1 g