
2-Chloro-9-cyclohexyl-N-[4-(4-Morpholinyl)phenyl]-9H-purin-6-aMine synthesis
- Product Name:2-Chloro-9-cyclohexyl-N-[4-(4-Morpholinyl)phenyl]-9H-purin-6-aMine
- CAS Number:737005-53-7
- Molecular formula:C21H25ClN6O
- Molecular Weight:412.92
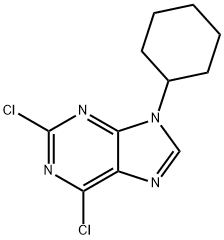
737005-47-9
1 suppliers
inquiry
2524-67-6
309 suppliers
$8.00/1g
![2-Chloro-9-cyclohexyl-N-[4-(4-Morpholinyl)phenyl]-9H-purin-6-aMine](/CAS/GIF/737005-53-7.gif)
737005-53-7
11 suppliers
$65.00/1mg
Yield:737005-53-7 66%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine in isopropanol at 120; for 3 h;
Steps:
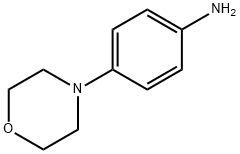
To a solution of Int-A1 (0.400 g, 1.47 mmol) in IPA (10 mL) wereadded 4-morpholinoaniline (0.262 g, 1.47 mmol) and N,N-diisopropylethylamine(1.02 mL, 5.9 mmol) at room temperature. Reactionmixture was allowed to stir at 120 C for 3h. The reaction mixture waspartitioned between water (75 mL) and EtOAc (75 mL). Organic layerwas separated out and dried over anhydrous Na2SO4. Solvent wasremoved in vacuum to obtain crude product which was purified bygradient column chromatography (normal phase, silica) product waseluted at 70% EtOAc in hexane to afford pure Int-A2 (0.400 g, 0.969mmol, 66%) as off white solid.LCMS: 100%, RT: 2.925 min, MS: 413.4 m/z [M+H]+; 415.4 m/z(M+3). 1H NMR (400 MHz, DMSO-d6) δ [ppm] = 10.09 (s, 1H), 8.39 (s,1H), 7.63 (d, J = 8.4 Hz, 2H), 6.94 (d, J = 8.8 Hz, 2H), 4.36-4.30 (m,1H), 4.33 (t, J = 4.0 Hz, 4H), 3.10-3.06 (m, 4H), 2.02-1.99 (m, 2H),1.91-1.83 (m, 4H), 1.72-1.68 (m, 1H), 1.49-1.40 (m, 2H), 1.29-1.22(m, 1H).
References:
Singh, Kailash;Nawabjan, Shaik Abdullah;Zhang, Li;El-Nezami, Hani;Annapureddy, Rajasekar reddy;Chow, Billy KC. [European Journal of Medicinal Chemistry,2022,vol. 242,art. no. 114642]

5451-40-1
504 suppliers
$10.00/1g
![2-Chloro-9-cyclohexyl-N-[4-(4-Morpholinyl)phenyl]-9H-purin-6-aMine](/CAS/GIF/737005-53-7.gif)
737005-53-7
11 suppliers
$65.00/1mg

108-93-0
429 suppliers
$11.00/25g
![2-Chloro-9-cyclohexyl-N-[4-(4-Morpholinyl)phenyl]-9H-purin-6-aMine](/CAS/GIF/737005-53-7.gif)
737005-53-7
11 suppliers
$65.00/1mg