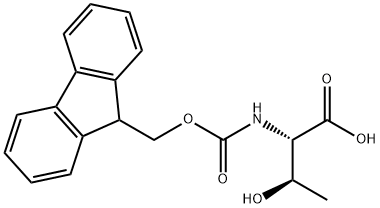
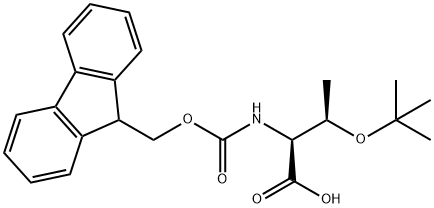
2-(9H-Fluoren-9-ylmethoxycarbonylamino)-3-hydroxy-butanoic acid synthesis
- Product Name:2-(9H-Fluoren-9-ylmethoxycarbonylamino)-3-hydroxy-butanoic acid
- CAS Number:73731-37-0
- Molecular formula:C19H19NO5
- Molecular Weight:341.36
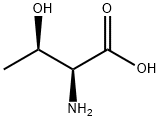
72-19-5
683 suppliers
$4.76/25g
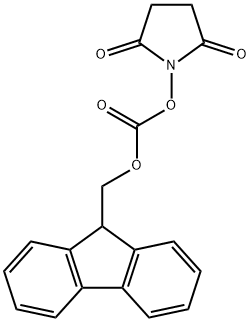
82911-69-1
609 suppliers
$7.00/25g

73731-37-0
293 suppliers
$13.00/1g
Yield:73731-37-0 100%
Reaction Conditions:
Stage #1:L-threonine;N-(9H-fluoren-2-ylmethoxycarbonyloxy)succinimide with sodium carbonate in 1,4-dioxane;water at 20;Inert atmosphere;
Stage #2: with hydrogenchloride in water; pH=4
Steps:
(2S,3R)-Benzyl 2-amino-3-((triisopropylsilyl)oxy)butanoate (17d)
At r.t. a solution of FmocONSu (3.37 g, 10.0 mmol) in 25 mL of Dioxane12 is added over 1h to a solution of L-threonine (1.19 g, 10.0 mmol) and Na2CO3 (1.06 g, 10.0 mmol) in 10 mL Dioxane and 25 mL water. The resulting suspension is stirred overnight and becomes clear. The solvent is evaporated in vacuo and the product is precipitated with 1M HClaq. at pH 4, filtered, washed with water and dissolved in EtOAc. The organic phase is dried (Na2SO4) and evaporated to yield the product quantitatively as a white solid which does not need any further purification. Under argon atmosphere Fmoc-threonine (2.67 g, 7.80 mmol) is dissolved in 32 mL dry MeOH. Then Cs2CO3 (1.40 g, 4.30 mmol) is added.11 After 15 min the solvent is evaporated and the resulting solid is three times suspended in CH2Cl2 and evaporated to remove all the MeOH. The Cs-salt is then suspended in 36 mL of dry DMF under argon atmosphere and stirred with benzylbromide (1.02 mL, 1.47 g, 8.60 mmol) over night at r.t. After evaporation of the solvent in vacuo, the residue is partitioned between water and CH2Cl2. The water phase is subsequently extracted two times with EtOAc. The combined organic phases are dried (Na2SO4) and evaporated. The crude product is purified either by recrystallisation from EtOAc or by silica column chromatography (cyclohexane/EtOAc 4 : 1) to get Fmoc-threoninebenzylester (3.07 g, 7.10 mmol, 91%, Rf = 0.17 (cyclohexane/EtOAc 3 : 1)) as a white solid. The Fmoc-threoninebenzylester (2.16 g, 5.00 mmol) is dissolved in 48 mL dry CH2Cl2 under argon atmosphere. The solution is cooled to 0°C and NEt3 (770 µL, 560 mg, 5.50 mmol) and Triisopropylsilyltriflat (1.42 mL, 1.61 g, 5.25 mmol) is added subsequently.12 The mixture is allowed to warm to r.t. and is stirred 1h. Then 30 mL of dilute K2CO3- solution is added. The phases are separated and the water phase is extracted two times with CH2Cl2. The combined organic phases are dried (Na2SO4) and evaporated. The crude product is purified by silica column chromatography (cyclohexane/EtOAc 10 : 1) to get Fmoc(OTIPS)benzylester (2.47 g, 4.20 mmol, 84%, Rf = 0.61 (cyclohexane/EtOAc 3 : 1)) as a colourless oil. Fmoc(OTIPS)benzylester (0.87 g, 1.48 mmol) is dissolved in 7.4 mL dry CH2Cl2 under argon atmosphere. The solution is cooled to 0°C and 7.4 mL of 40% (v/v) Piperidine solution in dry CH2Cl2 are added dropwise.13 After 15 min of stirring at 0°C, the mixture is concentrated in vacuo at 25°C to an oil and put immediately onto the silica column (cyclohexane/EtOAc 5 : 1 + 0.5% NEt3, elution of product with 1 : 1 + 0.5% NEt3). Yield of the title compound 17d is 0.44 g (1.20 mmol, 81%) as a colourless oil.
References:
Loke, Inga;Park, Natja;Kempf, Karl;Jagusch, Carsten;Schobert, Rainer;Laschat, Sabine [Tetrahedron,2012,vol. 68,# 2,p. 697 - 704] Location in patent:supporting information; experimental part
176380-53-3
160 suppliers
$5.00/100mg

73731-37-0
293 suppliers
$13.00/1g

72-19-5
683 suppliers
$4.76/25g

28920-43-6
528 suppliers
$5.00/5g

73731-37-0
293 suppliers
$13.00/1g
71989-35-0
435 suppliers
$8.00/5g

73731-37-0
293 suppliers
$13.00/1g
![methyl (2S,3R)-2-({[(9H-fluoren-9-yl)methoxy]carbonyl}amino)-3-hydroxybutanoate](/CAS/20210111/GIF/89024-98-6.gif)
89024-98-6
1 suppliers
inquiry

73731-37-0
293 suppliers
$13.00/1g