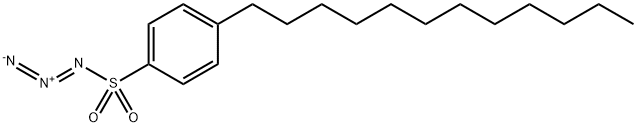
2-Azetidinebutanoic acid, a-diazo-3-(1-hydroxyethyl)-b,4-dioxo-, (4-nitrophenyl)Methyl ester, [2R-[2a,3b(R*)]]- synthesis
- Product Name:2-Azetidinebutanoic acid, a-diazo-3-(1-hydroxyethyl)-b,4-dioxo-, (4-nitrophenyl)Methyl ester, [2R-[2a,3b(R*)]]-
- CAS Number:74288-39-4
- Molecular formula:C16H16N4O7
- Molecular Weight:376.33
Yield:74288-39-4 222 mg, (81% overall from (3S,4R)-1-(t-butyldimethylsilyl)-3-[(R)-1-(t-butyl dimethylsilyloxy)ethyl]-4-[3-(4-nitrobenzyl)oxycarbonyl-2-oxopropyl]azetidin-2-one)
Reaction Conditions:
with triethylamine in acetonitrile;
Steps:
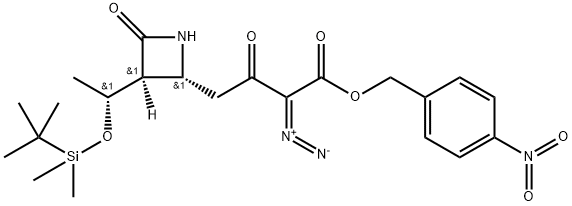
14 Preparation of (3S,4R)-3-[(R)-1-hydroxyethyl]-4-[3-(4-nitrobenzyl)oxycarbonyl-2-oxo-3-diazopropyl]azetidin-2-one STR82
EXAMPLE 14 Preparation of (3S,4R)-3-[(R)-1-hydroxyethyl]-4-[3-(4-nitrobenzyl)oxycarbonyl-2-oxo-3-diazopropyl]azetidin-2-one STR82 Triethylamine(263 mg, 2.6 mmol) is added by syringe to a mixture of (3S,4R)-3-[(R)-1-hydroxyethyl]-4-[3-(4-nitrobenzyl)oxycarbonyl-2-oxopropyl]azetidin-2-one(253 mg, 0.72 mmol) and p-carboxybenzene sulfonylazide (196 mg, 0.84 mmol) in dry acetonitrile (6 ml) at 0° C. When addition is complete the cooling bath is removed and the reaction mixture is stirred at room temperature for 1 hour. The mixture is then diluted with ethyl acetate (50 ml) and filtered. The filtrate is concentrated in vacuo and the residue is chromatographed on a short silica gel column (ethyl acetate) to yield 222 mg, (81% overall from (3S,4R)-1-(t-butyldimethylsilyl)-3-[(R)-1-(t-butyl dimethylsilyloxy)ethyl]-4-[3-(4-nitrobenzyl)oxycarbonyl-2-oxopropyl]azetidin-2-one) of (3S,4R)-3-(R)-1-hydroxyethyl)-4-[3-(4-nitrobenzyl)oxycarbonyl-2-oxo-3-diazopropyl]azetidin-2-one as a white solid m.p. (dec.) 163°
References:
US4309346,1982,A
93861-39-3
1 suppliers
inquiry
![2-Azetidinebutanoic acid, a-diazo-3-(1-hydroxyethyl)-b,4-dioxo-, (4-nitrophenyl)Methyl ester, [2R-[2a,3b(R*)]]-](/CAS/20211123/GIF/74288-39-4.gif)
74288-39-4
5 suppliers
inquiry
![2-Azetidinebutanoic acid, 3-[(1R)-1-hydroxyethyl]-β,4-dioxo-, (4-nitrophenyl)methyl ester, (2R,3S)-](/CAS/20210305/GIF/75321-07-2.gif)
75321-07-2
0 suppliers
inquiry
![2-Azetidinebutanoic acid, a-diazo-3-(1-hydroxyethyl)-b,4-dioxo-, (4-nitrophenyl)Methyl ester, [2R-[2a,3b(R*)]]-](/CAS/20211123/GIF/74288-39-4.gif)
74288-39-4
5 suppliers
inquiry
![3-Butenoic acid, 2-diazo-3-[[(1,1-dimethylethyl)dimethylsilyl]oxy]-, (4-nitrophenyl)methyl ester](/CAS/20210111/GIF/93788-48-8.gif)
93788-48-8
3 suppliers
inquiry
![2-Azetidinebutanoic acid, a-diazo-3-(1-hydroxyethyl)-b,4-dioxo-, (4-nitrophenyl)Methyl ester, [2R-[2a,3b(R*)]]-](/CAS/20211123/GIF/74288-39-4.gif)
74288-39-4
5 suppliers
inquiry