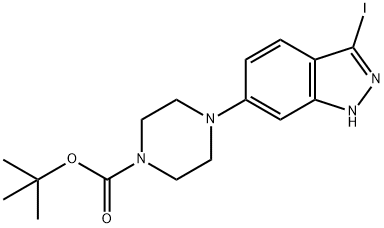
Tert-Butyl 4-(3-iodo-1H-indazol-6-yl)piperazine-1-carboxylate synthesis
- Product Name:Tert-Butyl 4-(3-iodo-1H-indazol-6-yl)piperazine-1-carboxylate
- CAS Number:744219-44-1
- Molecular formula:C16H21IN4O2
- Molecular Weight:428.27
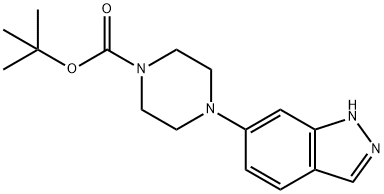
744219-43-0
34 suppliers
$52.00/50mg

744219-44-1
29 suppliers
$293.18/250mg
Yield:744219-44-1 38%
Reaction Conditions:
with potassium hydroxide;iodine in DMF (N,N-dimethyl-formamide) at 0 - 20;
Steps:
116 EXAMPLE 116; Preparation of 6-[4-(t-Butoxycarbonyl)piperazin-1-yl]-3-iodo-1H-indazole
EXAMPLE 116 Preparation of 6-[4-(t-Butoxycarbonyl)piperazin-1-yl]-3-iodo-1H-indazole A stirred solution of 6-[4-(t-butoxycarbonyl)piperazin-1-yl]-1H-indazole (7.20 g, 23.8 mmol) in N,N-dimethylformamide at 0° C. is treated with powdered potassium hydroxide (5.40 g, 95.4 mmol) followed by the dropwise addition of a solution of iodine (10.9 g, 42.9 mmol) in N,N-dimethylformamide, stirred at ambient temperatures for 16 h, diluted with ethyl acetate and quenched with 10% aqueous sodium metabisulfite solution.The phases are separated.The aqueous phase is extracted with ethyl acetate.The extracts are combined with the organic phase, washed with brine, dried over sodium sulfate and concentrated in vacuo.The resulting crude material is purified by flash chromatography (silica gel, 30:70 ethyl acetate/hexanes) to afford the title compound as a yellow solid, 3.4 g (38% yield) identified by NMR and mass spectral analyses.
References:
US2004/167122,2004,A1 Location in patent:Page/Page column 23