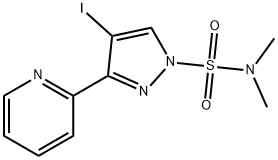
1H-Pyrazole-1-sulfonaMide, 4-iodo-N,N-diMethyl-3-(2-pyridinyl)- synthesis
- Product Name:1H-Pyrazole-1-sulfonaMide, 4-iodo-N,N-diMethyl-3-(2-pyridinyl)-
- CAS Number:746668-78-0
- Molecular formula:C10H11IN4O2S
- Molecular Weight:378.19
Yield:746668-78-0 49%
Reaction Conditions:
with triethylamine in chloroform at 0;Heating / reflux;
Steps:
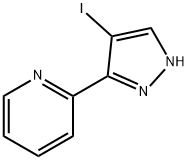
1.c (C) 4-IODO-3-PYRIDIN-2-YL-PYRAZOLE-1-SULFONIC acid dimethylamide
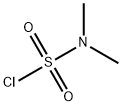
To a solution of 2. 46 g (9. 1 mmol) OF 2- (4-IODO-LH-PYRAZOL-3-YL)-PYRIDINE in 100 mL CHCl3 was added 7. 0 mL (50 mmol, 5. 5 equiv.) of triethylamine with stirring to give a pale yellow solution. This was cooled to 0 C and 4. 9 mL (45. 4 mmol, 5 equiv.) OF N, N-DIMETHYLSULFAMOYL chloride was added slowly over 10 minutes. The yellow solution was allowed to wann to room temperature, then heated to reflux overnight with stirring. The resulting solution was cooled, washed twice with IN NAOH, then brine, and dried, filtered and concentrated. The residue was dissolved in about 50 mL of 1 : 1 ethyl ACETATE/HEXANES, passed through a 1.5 inch silica gel plug. The silica plug was washed with an additional 200 mL of 1 : 1 EA/hex to give a pale orange filtrate. The filtrate was concentrated and the orange residue recrystallized from ethanol/water to give 1. 67 g (4. 4 mmol, 49%) of 4-iodo-3-pyridin-2-yl-pyrazole-1- sulfonic acid dimethylamide as fine, light orange CRYSTALS. 1H-NMR (300 MHz, CDC13, 8) : 8. 74 (dq, J = 0. 9 Hz, 1. 8 Hz, 4. 8 Hz, 1 H), 8. 11 (S, 1 H), 7. 95 (dt, J = 1. 2 Hz, 7. 8 Hz, 1 H), 7. 77 (td, J = 1. 8 Hz, 7. 5 Hz, 1 H), 7. 33 (qd, J = 1. 2 Hz, 4. 8 Hz, 1 H), 3. 00 (s, 6H) ; M/Z : 379 [M + H] +.
References:
WO2004/72033,2004,A2 Location in patent:Page 36