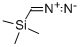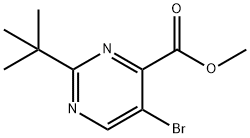
Methyl 5-bromo-2-(tert-butyl)pyrimidine-4-carboxylate synthesis
- Product Name:Methyl 5-bromo-2-(tert-butyl)pyrimidine-4-carboxylate
- CAS Number:746671-54-5
- Molecular formula:C10H13BrN2O2
- Molecular Weight:273.13
Yield:746671-54-5 81%
Reaction Conditions:
in methanol;hexane;benzene; for 48 h;
Steps:
D5.1
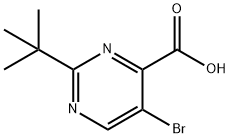
A 2.0 M hexanes solution of trimethylsilyldiazomethane (11.8 mL, 23.62 mMol) was added dropwise to a stirring solution of 5-bromo-2-tert-butyl-pyrimidine-4-carboxylic acid (6.12 g, 23.62 mMol) in 9:1 benzene/methanol (100 mL), and the reaction was stirred for 2 days. TLC analysis showed that the reaction was complete, so the mixture was concentrated in-vacuo. The residue was dissolved in ethyl acetate (100 mL), washed with water (3*20 mL), dried over sodium sulfate, then concentrated in-vacuo. Purified over silica gel, eluding with 10% ethyl acetate/hexanes, to yield 5.2 g of a colorless oil as product. MS (ES+)=273,275 (M+H)+. Yield=81%.
References:
US2005/54627,2005,A1 Location in patent:Page/Page column 42