
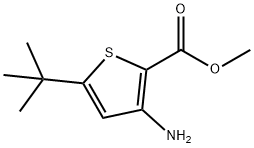
2-Thiophenecarboxylicacid,3-amino-5-(1,1-dimethylethyl)-(9CI) synthesis
- Product Name:2-Thiophenecarboxylicacid,3-amino-5-(1,1-dimethylethyl)-(9CI)
- CAS Number:746671-67-0
- Molecular formula:C9H13NO2S
- Molecular Weight:199.27
175137-03-8
63 suppliers
$16.00/250mg

746671-67-0
14 suppliers
$300.00/5g
Yield:-
Reaction Conditions:
with water;potassium hydroxide in methanol at 80; for 4.5 h;
Steps:
6-tert-butyl-1H-thieno[2,3-d][1,3]oxazine-2,4-dione (5)
To a vial containing methyl 5-tert-butyl-2-aminothiophene-3-carboxylate, 3, (639.6 mg, 3 mmol) in 1:1 methanol:water (6 mL) was added KOH (496.4 mg, 8.8 mmol). The vial was capped and the mixture was stirred at 80 °C until no starting material was seen by TLC (4.5 h) The solvent was removed under vacuum, and the crude product was transferred to a 100 mL round bottom flask using water (50 mL). To the vigorously stirred aqueous solution was added phosgene (2M in toluene, 6 mL, 11 mmol) dropwise over a six minute period. The resulting mixture was stirred at room temperature overnight. The mixture was filtered, and the gummy solids were washed with water then dissolved in ethyl acetate (EtOAc). The organic solution was dried over anhydrous sodium sulfate, filtered and the solvent was removed under vacuum to give 626.9 mg (93%) of 5 as a tan solid. The material was used without further purification.
References:
Moffett, Kristofer;Konteatis, Zenon;Nguyen, Duyan;Shetty, Rupa;Ludington, Jennifer;Fujimoto, Ted;Lee, Kyoung-Jin;Chai, Xiaomei;Namboodiri, Haridasan;Karpusas, Michael;Dorsey, Bruce;Guarnieri, Frank;Bukhtiyarova, Marina;Springman, Eric;Michelotti, Enrique [Bioorganic and Medicinal Chemistry Letters,2011,vol. 21,# 23,p. 7155 - 7165] Location in patent:supporting information; experimental part