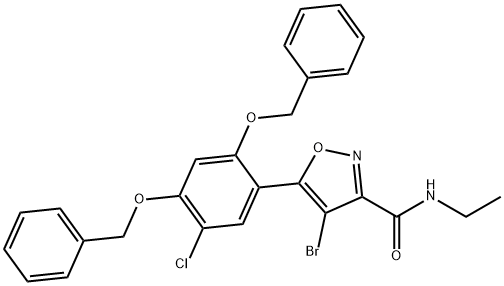
3-IsoxazolecarboxaMide, 4-broMo-5-[5-chloro-2,4-bis(phenylMethoxy)phenyl]-N-ethyl- synthesis
- Product Name:3-IsoxazolecarboxaMide, 4-broMo-5-[5-chloro-2,4-bis(phenylMethoxy)phenyl]-N-ethyl-
- CAS Number:747413-06-5
- Molecular formula:C26H22BrClN2O4
- Molecular Weight:541.82
![3-IsoxazolecarboxaMide, 5-[5-chloro-2,4-bis(phenylMethoxy)phenyl]-N-ethyl-](/CAS/GIF/747413-05-4.gif)
747413-05-4
6 suppliers
inquiry
![3-IsoxazolecarboxaMide, 4-broMo-5-[5-chloro-2,4-bis(phenylMethoxy)phenyl]-N-ethyl-](/CAS/GIF/747413-06-5.gif)
747413-06-5
4 suppliers
inquiry
Yield:747413-06-5 85.4%
Reaction Conditions:
with bromine;potassium acetate;acetic acid at 20; for 0.0833333 h;
Steps:
1h
Step 1h: 5-(2,4-Bis(benzyloxy)-5-chlorophenyl)-4-bromo-N-ethyl-isoxazole-3-carboxamide (Compound 0108) A solution of bromine in acetic acid (0.6 M, 306.0 ml, 183.6 mmol) was added to a stirred suspension of 0107 (8.50 g, 18.36 mmol) and potassium acetate (3.97 g, 40.50 mmol) in acetic acid (127 ml) at room temperature. The mixture was stirred at room temperature for 5 min. And saturated solution of Na2SO3 was added to the solution. After the mixture was concentrated to near dry, water (50 mL) was added and the mixture was filtered, the solid was washed with water and cooled ethanol (20 ml) and dried to obtain compound 0108 as a white solid (8.50 g, 85.4%): LCMS: 543 [M+1]+. 1H NMR (CDCl3): δ 1.26 (t, J=6 Hz, 3H), 3.45-3.54 (m, 2H), 5.06 (s, 2H), 5.11 (s, 2H), 6.61 (s, 1H), 6.73 (t, J=6 Hz, 1H), 7.25-7.39 (m, 10H), 7.52 (s, 1H).
References:
US2009/76006,2009,A1 Location in patent:Page/Page column 28; 35