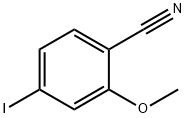
2-methoxy-4-(tetramethyl-1,3,2-dioxaborolan-2-yl)benzonitrile synthesis
- Product Name:2-methoxy-4-(tetramethyl-1,3,2-dioxaborolan-2-yl)benzonitrile
- CAS Number:755030-94-5
- Molecular formula:C14H18BNO3
- Molecular Weight:259.11
Yield:755030-94-5 65%
Reaction Conditions:
with potassium acetate;(1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride in dimethyl sulfoxide at 80; for 2 h;
Steps:
267.267C Example 267; 2-[3-(4-cyano-3-methoxyphenyl)-11-oxo-10,11-dihydro-5H-dibenzo[b,e][1,4]diazepin-8-yl]-2-methyl-N-(4-morpholin-4-ylphenyl)propanamide; Example 267C; 2-methoxy-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzonitrile
Example 267B (2. 0g, 7.7 mmol), bis (pinacolato) -diboron, (2.2g, 8.7 mmol), KOAC (2.2g, 22.4 mmol), and PDCL2 (DPPF)-CH2CI2 (315 mg, 0.39 mmol) in DMSO (40 mL) was heated at 80 C for 2 hours. The reaction was then cooled, filtered through CELITE then partitioned between toluene and water. The organic layer was washed with brine, dried over NA2SO4, filtered and concentrated to give 2.3g crude material. That crude was stirred in 50 C hexane (35 mL) for 1 hour, filtered, and the filtrate was allowed to cool to room temperature, then placed in the refigerator overnight. Recovered 1.3g (65%) grayish-brown crystals. MS (DCI) M/E 277 (M+NH4) + ; 1H NMR (300 MHz, DMSO-D6) 8 7.73 (d, J=7.8 Hz, 1H), 7.35 (m, 2H), 3.93 (s, 3H), 1.32 (s, 12H).
References:
WO2004/76424,2004,A1 Location in patent:Page 181
330793-38-9
142 suppliers
inquiry

73183-34-3
566 suppliers
$6.00/5g

755030-94-5
18 suppliers
inquiry
6609-56-9
213 suppliers
$8.00/10g

73183-34-3
566 suppliers
$6.00/5g

755030-94-5
18 suppliers
inquiry
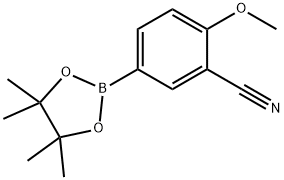
1220219-22-6
30 suppliers
inquiry