
3-(1-(2,6-dichloro-3-fluorophenyl)ethoxy)-2-nitropyridine synthesis
- Product Name:3-(1-(2,6-dichloro-3-fluorophenyl)ethoxy)-2-nitropyridine
- CAS Number:756521-08-1
- Molecular formula:C13H9Cl2FN2O3
- Molecular Weight:331.13
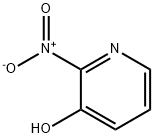
15128-82-2
433 suppliers
$10.00/10g
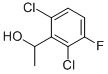
756520-66-8
91 suppliers
$10.00/1g

756521-08-1
15 suppliers
inquiry
Yield:756521-08-1 100%
Reaction Conditions:
with di-isopropyl azodicarboxylate;triphenylphosphine in tetrahydrofuran at 0 - 20; for 4 h;Inert atmosphere;
Steps:
1.2 Step 2 (±)3-(1-(2,6,-dichloro-3-fluorophenyl)ethoxy)-2-nitropyridine
Step 2 (±)3-(1-(2,6,-dichloro-3-fluorophenyl)ethoxy)-2-nitropyridine [00161] To a solution of triphenylphosphine (24.29 g, 92.6 mmol) in THF (dry, 160 ml_) was added DIAD (18.23 ml_, 92.6 mmol) dropwise at 0 °C under N2. After addition of DIAD, a solution of 1-(2,6-dichloro-3-fluorophenyl)ethanol (13.35 g, 63.86 mmol) and 3-hydroxy-2-nitropyridine (10.29 g, 73.44 mmol) in THF (anhydrous, 160 ml_) was added dropwise. The ice-bath was removed and the reaction mixture was allowed to warm to room temperature and stirred at room temperature for 4 hrs. The reaction mixture was concentrated by evaporator to give a yellow residue. A saturated solution of NH4CI (200 ml_) was added. The aqueous phase was extracted with EtOAc (3^150 mL). The combined organic phase was washed with brine (2*80 mL), dried over anhydrous Na2S04, concentrated by evaporation in vacuo to give a yellow residue which was purified by CombiFlash (220 g silica gel column, Hexane/EtOAc) to afford (±)-3-(1-(2,6,- dichloro-3-fluorophenyl)ethoxy)-2-nitropyridine (21.1 g, 100%) as a white solid. [00162] 1HNMR (300 MHz, CDCI3): δ 8.04 (d, 1 H), 7.37 (m, 1 H), 7.30 (dd, 1 H), 7.21 (d, 1 H), 7.09 (t, 1 H), 6.10 (q, 1 H), 1.85 (d, 3 H).
References:
WO2013/13308,2013,A1 Location in patent:Paragraph 00160-00162

15128-82-2
433 suppliers
$10.00/10g
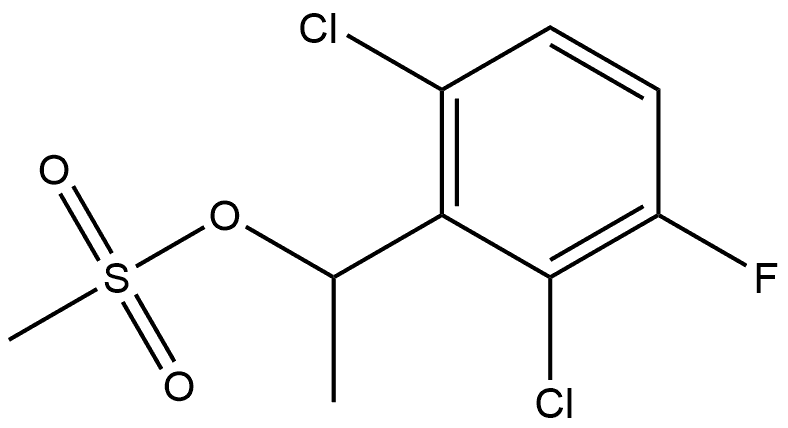
1229457-91-3
0 suppliers
inquiry

756521-08-1
15 suppliers
inquiry
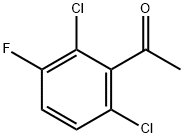
290835-85-7
262 suppliers
$6.00/10g

756521-08-1
15 suppliers
inquiry