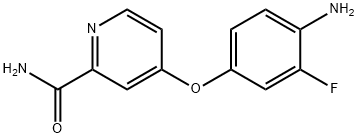
4-(4-AMino-3-fluorophenoxy)-pyridine-2-carboxylic acid aMide synthesis
- Product Name:4-(4-AMino-3-fluorophenoxy)-pyridine-2-carboxylic acid aMide
- CAS Number:757251-54-0
- Molecular formula:C12H10FN3O2
- Molecular Weight:247.23
99586-65-9
234 suppliers
$14.00/5g
399-95-1
402 suppliers
$14.00/5g

757251-54-0
15 suppliers
inquiry
Yield:757251-54-0 82.3%
Reaction Conditions:
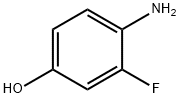
Stage #1: 4-amino-3-fluorophenolwith potassium tert-butylate in tetrahydrofuran;dimethyl sulfoxide at 10 - 20; for 0.166667 h;Inert atmosphere;Large scale;
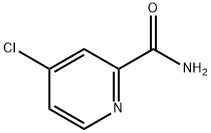
Stage #2: 4-chloropicolinamide in tetrahydrofuran;dimethyl sulfoxide at 80 - 85; for 2.66667 h;Large scale;Williamson Ether Synthesis;Temperature;
Steps:
1; 3; 5 Example 1 Preparation of 2-carbamoyl-4-((3-fluoro-4-amino)phenoxy)pyridine (Compound 4)
Add 19.36 kg of dimethyl sulfoxide and 7.2 kg of tetrahydrofuran into a 100L reactor,Blow in nitrogen protection, then add 4.88 kg potassium tert-butoxide,After it is completely dissolved, control the temperature at 10-20°C.Continue to add 4.54 kg 4-amino-3-fluorophenol (compound 3),Stir at room temperature for 10 minutes,Add 5.57 kg 4-chloro-2-pyridinecarboxamide (compound 2),Continue to stir for 10 minutes,The temperature of the reaction solution was raised to above 80°C within 30 minutes.Start timing when the internal temperature rises to 80,Incubate the reaction at 85±2°C for 2.0 hours.After the reaction, cool down to below 20°C,Drop 70 kg of 1M sodium hydroxide aqueous solution,After dripping, the temperature in the reactor is slowly reduced to 0-2°C.The slurry was filtered while it was cold, and the filter cake was washed with 120 kg of deionized water.The filter cake was suspended in 80 kg deionized water for 30 minutes and filtered.The filter cake was washed with 20 kg deionized water and dried under reduced pressure to obtain Intermediate 4.It weighs 6.5 kg (the yield is about 82.3%).
References:
CN112159351,2021,A Location in patent:Paragraph 0025; 0027; 0029

99586-65-9
234 suppliers
$14.00/5g

757251-54-0
15 suppliers
inquiry