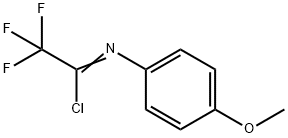
2,2,2-TRIFLUORO-N-(4-METHOXY-PHENYL)-ACETIMIDOYL CHLORIDE synthesis
- Product Name:2,2,2-TRIFLUORO-N-(4-METHOXY-PHENYL)-ACETIMIDOYL CHLORIDE
- CAS Number:75999-66-5
- Molecular formula:C9H7ClF3NO
- Molecular Weight:237.61
Yield:75999-66-5 89%
Reaction Conditions:
Stage #1: trifluoroacetic acidwith tetrachloromethane;triethylamine;triphenylphosphine in tetrachloromethane; for 0.166667 h;Cooling with ice;
Stage #2: 4-methoxy-aniline in tetrachloromethane; for 4 h;Reflux;
Steps:
1 Example 1
2,2,2-Trifluoro-N-(4-methoxyphenyl)acetimidoyl chloride
A mixture of triphenylphosphine (34.6 grams (g), 132.0 millimoles (mmol)), 2,2,2-trifluoroacetic acid (3.37 milliliters (mL), 44 mmol), triethylamine (7.38 mL, 53.0 mmol) and carbon tetrachloride (21.33 mL, 220.0 mmol) was magnetically stirred while cooled with an ice bath.
After 10 minutes (min), p-methoxyaniline (6.53 g, 53.0 mmol) dissolved in carbon tetrachloride (21.33 mL, 220.0 mmol) was added slowly (exothermic).
The ice bath was removed and the reaction mixture was stirred at reflux for 4 hours (h).
Upon cooling to room temperature, the reaction mixture was washed with hexane (3*100 mL).
Solvent was removed using a rotary evaporator to give 9.8 g of an orange oil.
Distillation gave 2,2,2-trifluoro-N-(4-methoxyphenyl)acetimidoyl chloride (9.31 g, 39.2 mmol, 89% yield) as a light yellow liquid: bp 75-77° C./0.3 mmHg; 1H NMR (400 MHz, CDCl3) δ 7.31 (m, 2H), 6.96 (m, 2H), 3.84 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 159.56 (s), 135.45 (s), 127.98 (q), 124.35 (s), 117.05 (q), 114.25 (s), 55.50 (s).
References:
US2014/171653,2014,A1 Location in patent:Paragraph 0059; 0060

332-34-3
23 suppliers
$12.00/1mg

75999-66-5
33 suppliers
$269.00/5g