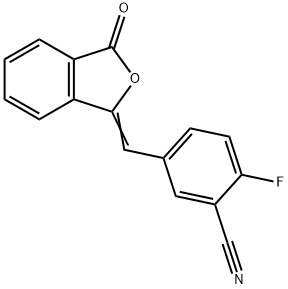
2-Fluoro-5-((3-oxoisobenzofuran-1(3H)-ylidene)methyl)benzonitrile synthesis
- Product Name:2-Fluoro-5-((3-oxoisobenzofuran-1(3H)-ylidene)methyl)benzonitrile
- CAS Number:763114-25-6
- Molecular formula:C16H8FNO2
- Molecular Weight:265.24
![2-Fluoro-5-[(3-oxo-1(3H)-isobenzofuranylidene)methyl]-benzonitrile 2-Fluoro-5-[(3-oxo-1(3H)-isobenzofuranylidene)methyl]-benzonitrile](/NewsImg/2023-10-18/6383321843554810432958503.jpg)
Yield:763114-25-6 202.3 g
Reaction Conditions:
with triethylamine for 1 h;
Steps:
4-6
2) 252.92 g of TEA was added to the prepared reaction solution containing compound III, 163.90 g of IIb was added in batches, and the reaction was carried out for 1 h. The reaction was monitored by HPLC, and the content of Compound III was less than 5% as the end of the reaction;The resulting reaction solution was suction filtered, rinsed with anhydrous methanol, the filter cake was added to 3000 ml of water, stirred, and the temperature was controlled at 30°C, and stirred for 1 h. Suction filtration, rinsed with water, and air-dried at 60°C to obtain compound IV 202.03g, yield 76.2%, purity 99.87%, total impurities 0.13%, unknown maximum single impurities 0.05%.
References:
CN114075159,2022,A Location in patent:Paragraph 0030; 0117-0136
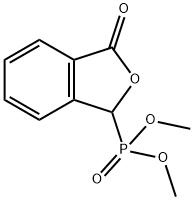
61260-15-9
181 suppliers
inquiry
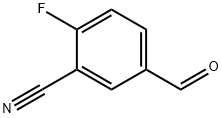
218301-22-5
377 suppliers
$10.00/1g

763114-25-6
211 suppliers
inquiry
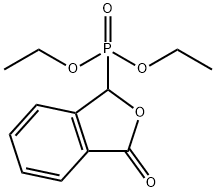
17858-15-0
8 suppliers
inquiry

218301-22-5
377 suppliers
$10.00/1g

763114-25-6
211 suppliers
inquiry
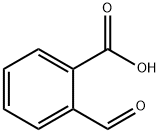
119-67-5
336 suppliers
$2.00/1g

218301-22-5
377 suppliers
$10.00/1g

763114-25-6
211 suppliers
inquiry

61260-15-9
181 suppliers
inquiry
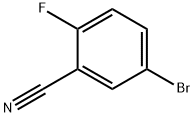
179897-89-3
313 suppliers
$10.00/5g

763114-25-6
211 suppliers
inquiry
