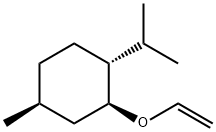
Cyclohexane, 2-(ethenyloxy)-4-methyl-1-(1-methylethyl)-, (1R,2S,4S)- synthesis
- Product Name:Cyclohexane, 2-(ethenyloxy)-4-methyl-1-(1-methylethyl)-, (1R,2S,4S)-
- CAS Number:7637-11-8
- Molecular formula:C12H22O
- Molecular Weight:182.3
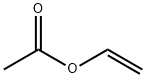
108-05-4
578 suppliers
$4.00/1000g

15356-60-2
173 suppliers
$30.00/20mg

7637-11-8
0 suppliers
inquiry
Yield:7637-11-8 87%
Reaction Conditions:
with bis(1,5-cyclooctadiene)diiridium(I) dichloride;sodium carbonate in toluene at 100; for 3 h;Inert atmosphere;
Steps:
General procedure for the synthesis of vinyl ethers (General Procedure I).
General procedure: A two-necked, 500-mL round bottom flask was equipped with a magnetic stirring bar and a three-way stopcock, of which one way was connected to an argon flow line and the other was connected to a vacuum line. The apparatus was flame-dried in vacuum, and allowed to cool to room temperature under an argon purge. To a solution of [IrCl(cod)]2 (1 mol%) in dry toluene triethylene glycol monomethyl ether, vinyl acetate (2.0 equiv), and Na2CO3 (0.60 equiv) were added and the mixture was stirred at 100 °C for 3 h. After the reaction, the resulting salts were removed and washed with Et2O. The solvent was removed under reduced pressure. Purification of the residue by vacuum distillation (2 times distillation with CaH2) gave the product.
References:
Harada, Nari-Aki;Nishikata, Takashi;Nagashima, Hideo [Tetrahedron,2012,vol. 68,# 15,p. 3243 - 3252] Location in patent:supporting information; experimental part

15356-60-2
173 suppliers
$30.00/20mg
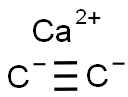
75-20-7
152 suppliers
$56.50/500g

7637-11-8
0 suppliers
inquiry

15356-60-2
173 suppliers
$30.00/20mg

7637-11-8
0 suppliers
inquiry

111-34-2
246 suppliers
$14.00/25mL

15356-60-2
173 suppliers
$30.00/20mg

7637-11-8
0 suppliers
inquiry