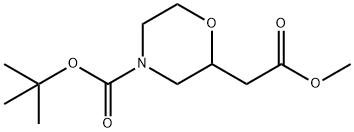
tert-butyl 2-(2-methoxy-2-oxoethyl)morpholine-4-carboxylate synthesis
- Product Name:tert-butyl 2-(2-methoxy-2-oxoethyl)morpholine-4-carboxylate
- CAS Number:766539-39-3
- Molecular formula:C12H21NO5
- Molecular Weight:259.3
473269-88-4
41 suppliers
$90.00/100mg

24424-99-5
821 suppliers
$13.50/25G

766539-39-3
12 suppliers
inquiry
Yield:766539-39-3 45%
Reaction Conditions:
with potassium carbonate in tetrahydrofuran at 20;
Steps:
F
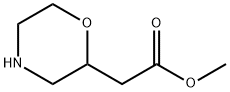
Intermediate F; Preparation of tert-butyl 2-(2-hvdroxyethyl)morpholine-4-carboxvlate; Methyl morpholin-2-yl acetate (5.0 g, 31.4 mmol) was diluted with THF (10 ml.) and water (10 ml.) and treated with potassium carbonate (4.34 g, 31.4 mmol). The thick suspension slowly went into solution. Di-terf-butyl dicarbonate (6.85 g, 31.4 mmol) was added and the reaction mixture was stirred at rt overnight. The reaction mixture was then extracted with THF and EtOAc. The organic layer was dried (MgSO4) and concentrated under reduced pressure. The sticky oil was triturated with ether and the resulting solid was collected by vacuum filtration (3.7 g, 45%). The mixture was diluted in THF (20 ml_) and treated with a solution of sodium hydroxide (2 N, 5 ml_) and stirred overnight. The reaction mixture was concentrated under reduced pressure and then diluted with water and EtOAc. The pH of the aqueous layer was adjusted to 5, and the organic layer was separated, dried (MgSO4) and concentrated under reduced pressure. The solid (2 g, 8.15
References:
WO2008/70150,2008,A1 Location in patent:Page/Page column 61-62