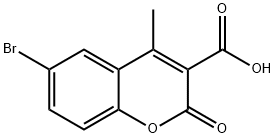
6-Bromo-4-methyl-2-oxo-2H-1-benzopyran-3-carboxylic acid synthesis
- Product Name:6-Bromo-4-methyl-2-oxo-2H-1-benzopyran-3-carboxylic acid
- CAS Number:773109-55-0
- Molecular formula:C11H7BrO4
- Molecular Weight:283.07
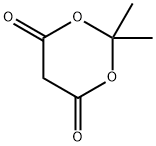
2033-24-1
645 suppliers
$6.00/25g
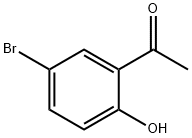
1450-75-5
301 suppliers
$10.00/1g

773109-55-0
11 suppliers
$3578.00/10g
Yield:773109-55-0 58%
Reaction Conditions:
Stage #1: 5-Bromo-2-hydroxyacetophenonewith ammonia in methanol at 0 - 20;
Stage #2: cycl-isopropylidene malonate in ethanol; for 5 h;Reflux;
Steps:
2.1.5 6-Bromo-4-methylcoumarin-3-carboxylic acid (UBP714)
A stirring solution of 5-bromo-2-hydroxyacetophenone (2) (9.95 g, 46 mmol) in methanol (80 mL) was cooled to 0 °C and ammonia gas bubbled through the solution until it was saturated. The resultant mixture was allowed to warm to room temperature, stirred for 18 h, and then concentrated in vacuo. Meldrum’s acid (7.95 g, 55.2 mmol) and ethanol (150 mL) were added to the residue and the resultant mixture heated under reflux for 5 h. The solution was then allowed to cool to room temperature during which time a solid precipitated out of solution. This was filtered off, washed thoroughly with ethanol, and dried in vacuo to afford UBP714 as a light yellow solid (7.55 g, 58%); mp: 201-203 °C; 1H NMR (400 MHz, DMSO-d6) δ 2.30 (s, 3H), 7.30 (d, J = 8.8 Hz, 1H), 7.67 (d, J = 8.8 and 2.4 Hz, 1H), 7.85 (d, J = 2.4 Hz, 1H), 13.32 (br s, 1H); 13C NMR (100 MHz, DMSO-d6) δ 15.5, 116.0, 118.2, 122.5, 127.4, 130.8, 132.7, 139.5, 150.6, 158.0, 166.4.
References:
Irvine, Mark W.;Costa, Blaise M.;Volianskis, Arturas;Fang, Guangyu;Ceolin, Laura;Collingridge, Graham L.;Monaghan, Daniel T.;Jane, David E. [Neurochemistry International,2012,vol. 61,# 4,p. 593 - 600]

56394-22-0
44 suppliers
$80.00/1g

773109-55-0
11 suppliers
$3578.00/10g

1450-75-5
301 suppliers
$10.00/1g

773109-55-0
11 suppliers
$3578.00/10g