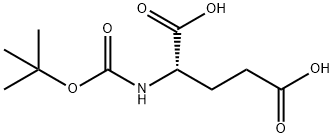
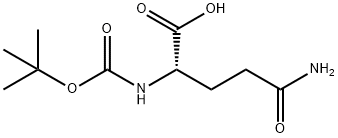
(S)-Tert-Butyl (1,5-diaMino-1,5-dioxopentan-2-yl)carbaMate synthesis
- Product Name:(S)-Tert-Butyl (1,5-diaMino-1,5-dioxopentan-2-yl)carbaMate
- CAS Number:77345-20-1
- Molecular formula:C10H19N3O4
- Molecular Weight:245.28
Yield:77345-20-1 97%
Reaction Conditions:
with pyridine;ammonium bicarbonate in tetrahydrofuran at 20; for 12 h;
Steps:
15
Example 15Synthesis of (25)-2-tert-Butoxycarbonylamino-pentanedioic acid diamide (compound 27b)A solution of N-Boc-L-glutamic acid (38.7 g, 156.5 mmol) in THF (450 mL) was added to successively pyridine (15.1 g, 190.9 mmol), Boc2O (91.2 g, 417.9 mmol), and ammonium bicarbonate (31.4 g, 397.2 mmol) with stirring. After 12 hours at room temperature, the reaction mixture was evaporated. The residue was triturated over t-BuOMe (500 mL) and the precipitate was collected by filtration and dried under vacuum to give compound 27b (37.3 g, 97%). MP: 130-132° C. (MeOH); 1H NMR (4d-MeOH, 300 MHz): δ1.45 (s, 9H), 1.85-1.90 (m, 1H), 2.04-2.07 (m, 1H), 2.31 (t, J=7.7 Hz, 2H), 4.02-4.05 (m, 1H); MS: m/e 268.1 (M++23).
References:
US2010/152452,2010,A1 Location in patent:Page/Page column 14
13726-85-7
391 suppliers
$10.00/5g

77345-20-1
1 suppliers
inquiry
![L-Glutamine, N2-[(1,1-dimethylethoxy)carbonyl]-, anhydride with 2-methylpropyl hydrogen carbonate (9CI)](/CAS/20240320/GIF/105888-44-6.gif)
105888-44-6
0 suppliers
inquiry

77345-20-1
1 suppliers
inquiry