Acetic acid, 2-(2-bromo-4-chlorophenoxy)-, 1,1-dimethylethyl ester synthesis
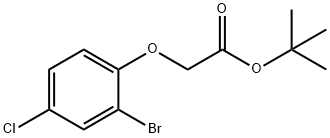
- Product Name:Acetic acid, 2-(2-bromo-4-chlorophenoxy)-, 1,1-dimethylethyl ester
- CAS Number:779328-99-3
- Molecular formula:C12H14BrClO3
- Molecular Weight:321.59
Yield:779328-99-3 95%
Reaction Conditions:
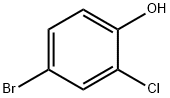
Stage #1: 2-bromo-4-chlorophenolwith potassium carbonate in acetone; for 0.166667 h;
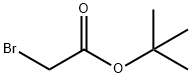
Stage #2: bromoacetic acid tert-butyl ester in acetone at 65; for 18 h;
Steps:
1
A solution of 2-bromo-4-chlorophenol (Aldrich; 13.11 g; 63.2 mmol) in acetone (100 mL) was treated with potassium carbonate (9.61 g; 69.5 mmol), stirred for 10 minutes then treated with te/f-butyl bromoacetate (Aldrich; 9.34 mL; 63.2 mmol). The reaction mixture was stirred at 65 0C for 18 hours, then the mixture was filtered, the solid was washed with acetone and the filtrate was concentrated to dryness under vacuum to give the title compound as a yellow sticky solid (19.4 g, 95%).1H NMR (300MHz, DMSOd6) δ [ppm] 7.68 (1 H, d, J= 2.6Hz), 7.39 (1 H, dd, J= 9.0 Hz; J= 2.6 Hz), 7.37 (1 H, d, J= 9.0 Hz), 4.80 (2H, s), 1.41 (9H, s). MS (ESI+): 340.1 (M+NH4+). HPLC (Condition A): Rt 5.06 min (HPLC purity 96.8%).
References:
WO2010/92043,2010,A1 Location in patent:Page/Page column 57