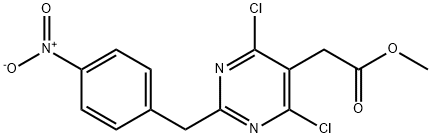
Methyl [4,6-dichloro-2-(4-nitrobenzyl)pyrimidin-5-yl]acetate synthesis
- Product Name:Methyl [4,6-dichloro-2-(4-nitrobenzyl)pyrimidin-5-yl]acetate
- CAS Number:780763-92-0
- Molecular formula:C14H11Cl2N3O4
- Molecular Weight:356.16
![[4,6-dihydroxy-2-(4-nitrobenzyl)pyrimidin-5-yl]acetic acid methyl ester](/CAS/20180703/GIF/780763-91-9.gif)
780763-91-9
0 suppliers
inquiry
![Methyl [4,6-dichloro-2-(4-nitrobenzyl)pyrimidin-5-yl]acetate](/CAS/GIF/780763-92-0.gif)
780763-92-0
28 suppliers
inquiry
Yield:780763-92-0 90%
Reaction Conditions:
with triethylamine;trichlorophosphate in toluene at 15 - 105; for 3.08333 h;
Steps:
1.E
Step E:To a stirred suspension of [4,6-dihydroxy-2-(4-nitrobenzyl)-pyrimidin-5-yl]-acetic acid methyl ester (200 g, obtained according to step F) in toluene (800 mL) in a 2500 mL double jacketed vessel at 20 +/- 5 °C is added 245 g POCI3. To this reaction mixture triethylamine (95 g) is added over a period of 5 minutes. The reaction mixture is heated to 103 +/- 2 °C (reflux conditions) and stirred for 3 hours. The excess POCI3 is removed in vacuo. The resulting suspension is cooled to 25 +/- 5°C and 800 mL of methanol is added. Subsequently an aqueous solution of NaOH (13% by weight, 164 g) is added and the reaction mixture is stirred at 20 +/- 5°C for 30 minutes. The solid is filtered off and washed subsequently with methanol (600 mL), deionized water (440 mL) and again with methanol (200 mL). The crystalline product is dried in vacuo at 30°C to yield [4,6-dichloro-2-(4-nitrobenzyl)pyrimidin- 5-yl]acetic acid methyl ester in an amount of 201 g (yield: 90%; HPLC-purity: 98,2%).
References:
WO2012/156221,2012,A1 Location in patent:Page/Page column 14
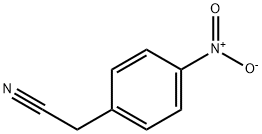
555-21-5
42 suppliers
$21.00/25g
![Methyl [4,6-dichloro-2-(4-nitrobenzyl)pyrimidin-5-yl]acetate](/CAS/GIF/780763-92-0.gif)
780763-92-0
28 suppliers
inquiry
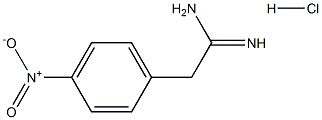
39695-96-0
9 suppliers
inquiry
![Methyl [4,6-dichloro-2-(4-nitrobenzyl)pyrimidin-5-yl]acetate](/CAS/GIF/780763-92-0.gif)
780763-92-0
28 suppliers
inquiry