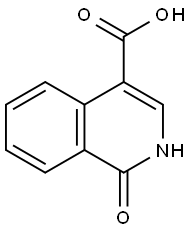
1-OXO-2-PHENYL-1,2-DIHYDRO-4-ISOQUINOLINECARBOXYLIC ACID synthesis
- Product Name:1-OXO-2-PHENYL-1,2-DIHYDRO-4-ISOQUINOLINECARBOXYLIC ACID
- CAS Number:78364-18-8
- Molecular formula:C16H11NO3
- Molecular Weight:265.26
Yield:78364-18-8 93%
Reaction Conditions:
with triethylamine in dichloromethane at 20;Molecular sieve;
Steps:
2 Synthesis of (S)-N-(2-(2-cyano-4,4-difluoropyrrolidin-1-yl)-2-oxoethyl)-1-oxo-2-phenyl-1,2-dihydroisoquinoline-4-carboxamide
Example 2
Synthesis of (S)-N-(2-(2-cyano-4,4-difluoropyrrolidin-1-yl)-2-oxoethyl)-1-oxo-2-phenyl-1,2-dihydroisoquinoline-4-carboxamide
Compound 2a:
To a stirred solution of 1-oxo-1,2-dihydroisoquinoline-4-carboxylic acid (500 mg, 2.6 mmol, 1 equiv) in DCM (10 mL), was added phenylboronic acid (480 mg, 3.9 mmol, 1.5 equiv), copper acetate (2360 mg, 13 mmol, 5 equiv), molecular sieves and Et3N (4 mL, 26 mmol, 10 equiv).
The reaction mixture was allowed to stir for overnight at RT.
Progress of the reaction was monitored by TLC and LCMS.
After completion of the reaction, the reaction mixture was passed through the Celite bed and the filtrate was concentrated under reduced pressure to obtain crude, which was diluted with water (100 mL) and washed with ethyl acetate (100 mL).
Aqueous layer was acidified with 3N HCl (30 mL) to pH-3 and extracted with ethyl acetate (100 mL*2).
Organic layer was dried over anhydrous sodium sulphate and concentrated under reduced pressure to obtain 1-oxo-2-phenyl-1,2-dihydroisoquinoline-4-carboxylic acid (650 mg, 93%) as a white solid.
References:
US2019/185451,2019,A1 Location in patent:Paragraph 0512-0514
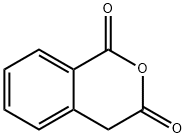
703-59-3
180 suppliers
$13.00/1g

78364-18-8
11 suppliers
inquiry
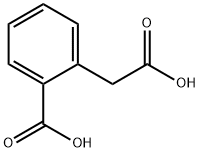
89-51-0
238 suppliers
$10.00/1g

78364-18-8
11 suppliers
inquiry

78364-23-5
0 suppliers
inquiry

78364-18-8
11 suppliers
inquiry
![1H-2-Benzopyran-1,3(4H)-dione, 4-[(phenylamino)methylene]-, (4Z)-](/CAS/20210305/GIF/73349-62-9.gif)
73349-62-9
0 suppliers
inquiry

78364-18-8
11 suppliers
inquiry