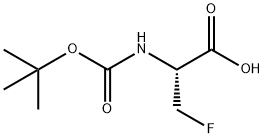
Alanine, N-[(1,1-dimethylethoxy)carbonyl]-3-fluoro- (9CI) synthesis
- Product Name:Alanine, N-[(1,1-dimethylethoxy)carbonyl]-3-fluoro- (9CI)
- CAS Number:78609-31-1
- Molecular formula:C8H14FNO4
- Molecular Weight:207.2
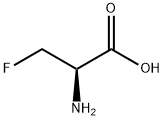
16652-37-2
28 suppliers
$320.00/100mg

24424-99-5
821 suppliers
$13.50/25G
![Alanine, N-[(1,1-dimethylethoxy)carbonyl]-3-fluoro- (9CI)](/CAS/GIF/78609-31-1.gif)
78609-31-1
4 suppliers
inquiry
Yield:78609-31-1 93%
Reaction Conditions:
with sodium carbonate in tetrahydrofuran;water at 20;
Steps:
134
A mixture of unprotected amino acid a (775 mg, 7.24 mmol) and sodium carbonate (1.69 g, 16.0 mmol) was dissolved in a 1:1 solution of deionized water and THF (15 mL each). To this mixture was added BOC-anhydride b (1.73 g, 7.96 mmol). The mixture was stirred at room temperature overnight, and THF was removed under reduced pressure. The mixture was then acidified to pH 2-3 with saturated aqueous citric acid, and product was extracted into 10% ethyl acetate-dichloromethane. The organic layer was dried (Na2SO4), filtered and concentrated under reduced pressure to afford clean BOC-protected amino acid c (1.40 g, 6.7 mmol, 93%) to be used without further purification.
References:
WO2006/69063,2006,A1 Location in patent:Page/Page column 134