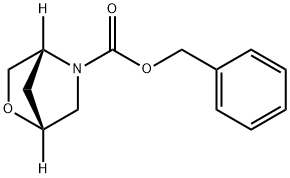
(1R,4R)-benzyl 2-oxa-5-azabicyclo[2.2.1]heptane-5-carboxylate synthesis
- Product Name:(1R,4R)-benzyl 2-oxa-5-azabicyclo[2.2.1]heptane-5-carboxylate
- CAS Number:787640-37-3
- Molecular formula:C13H15NO3
- Molecular Weight:233.26
![1-Pyrrolidinecarboxylic acid, 4-[[(1,1-dimethylethyl)dimethylsilyl]oxy]-2-[[(methylsulfonyl)oxy]methyl]-, phenylmethyl ester, (2R,4R)-](/CAS/20210305/GIF/787640-36-2.gif)
787640-36-2
0 suppliers
inquiry
![(1R,4R)-benzyl 2-oxa-5-azabicyclo[2.2.1]heptane-5-carboxylate](/CAS/20180703/GIF/787640-37-3.gif)
787640-37-3
10 suppliers
inquiry
Yield:787640-37-3 59%
Reaction Conditions:
Stage #1: (2R,4R)-benzyl 4-(tert-butyldimethylsilyloxy)-2-((methylsulfonyloxy)methyl)pyrrolidine-1-carboxylatewith tetrabutyl ammonium fluoride in tetrahydrofuran at 20; for 0.25 h;
Stage #2: with sodium hydride in tetrahydrofuran at 0 - 20; for 24 h;
Steps:
80.E Step E
A solution of the TBS-ether from the previous step (1.82 g, 4.10 mmol) in THF (50 mL) was treated with tetrabutylammonium fluoride (4.51 mL, 1 M solution in THF). And the reaction mixture was stirred at room temperature until LC MS analysis indicated complete removal of the TBS group, about 15 minutes. The reaction mixture was then cooled to 0 °C and sodium hydride (180 mg, 60 % suspension, 45.1 mmol) was added. Stirring at room temperature was continued for 24 h. The reaction was quenched by pouring onto water, and the crude product was extracted into ethyl acetate. The solvent was removed in vacuo, and the crude product was purified by flash chromatography (dichloromethane: ether/7: 3) to afford 595 mg (59 %) of the desired product.'H NMR (500 MHz, CDC13) : 7.38 (M, 5H), 5.30 (m, 2H), 4.52 (m, 2H), 3.80 (m, 2H), 3.40 (M, 2H), 1.70 (m, 2H).
References:
WO2004/94371,2004,A2 Location in patent:Page 130
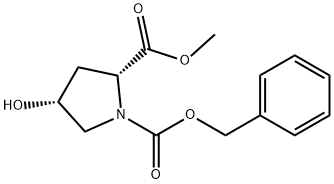
155075-23-3
74 suppliers
$29.00/250mg
![(1R,4R)-benzyl 2-oxa-5-azabicyclo[2.2.1]heptane-5-carboxylate](/CAS/20180703/GIF/787640-37-3.gif)
787640-37-3
10 suppliers
inquiry
![1,2-Pyrrolidinedicarboxylicacid-4-[[((1,1-diMethylethyl)diMethylsilyl]oxy]-2-Methyl-1-(phenylMethyl)ester,(2R,4R)](/CAS/20210305/GIF/787640-34-0.gif)
787640-34-0
0 suppliers
inquiry
![(1R,4R)-benzyl 2-oxa-5-azabicyclo[2.2.1]heptane-5-carboxylate](/CAS/20180703/GIF/787640-37-3.gif)
787640-37-3
10 suppliers
inquiry
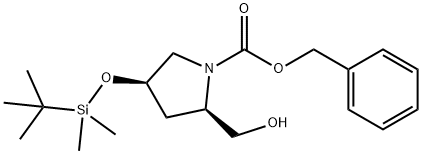
787640-35-1
1 suppliers
inquiry
![(1R,4R)-benzyl 2-oxa-5-azabicyclo[2.2.1]heptane-5-carboxylate](/CAS/20180703/GIF/787640-37-3.gif)
787640-37-3
10 suppliers
inquiry
![2-Oxa-5-azabicyclo[2.2.1]heptan-3-one, 5-acetyl-, (1R,4R)- (9CI)](/CAS/GIF/444313-67-1.gif)
444313-67-1
5 suppliers
inquiry
![(1R,4R)-benzyl 2-oxa-5-azabicyclo[2.2.1]heptane-5-carboxylate](/CAS/20180703/GIF/787640-37-3.gif)
787640-37-3
10 suppliers
inquiry