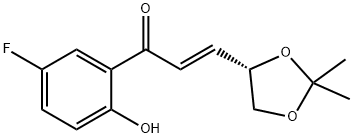
(E)-(4R)-4,5-Isopropylidene-dioxy-1-(2-hydroxy-5-fluorophenyl)propenone synthesis
- Product Name:(E)-(4R)-4,5-Isopropylidene-dioxy-1-(2-hydroxy-5-fluorophenyl)propenone
- CAS Number:797054-16-1
- Molecular formula:C14H15FO4
- Molecular Weight:266.26
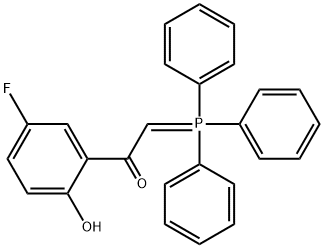
797054-15-0
8 suppliers
inquiry
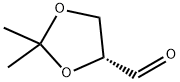
15186-48-8
319 suppliers
$8.00/5g

797054-16-1
9 suppliers
$120.00/250mg
Yield:-
Reaction Conditions:
in dichloromethane;
Steps:
8 Example 8; (E)-(4R)-4,5-isopropylydene-dioxy-1-(2-hydroxy-5-fluoro-phenyl)-prop-2E-ene-1-one (IX-R compound)
60 ml of saturated sodium hydrogen carbonate solution is added to a solution of 159 g (0,61moles) 1, 2: 5, 6-DI-0-ISOPROPYLYDENE- D-mannitole in 1 1 of dichloromethane, then 148 g (0,7moles) of solid sodium PERIODATE is added to the obtained dispersion under stirring in a rate which permits the temperature of reaction mixture is kept under 30°C. The reaction mixture is stirred for additional 30 minutes, 100 g of anhydrous magnesium sulphate is added, the mixture is stirred additional 10 minutes, filtered. The given 1200 ml solution OF D (+) R- O, O-ISOPROPYLYDENE-GLYCERINEALDEHYDE (VIII-R) is stirred and 251 g (0,61 moles) of 1-(5-FLUORO-2-HYDROXY-PHENYL)-2-(TRIPHENYL-S 5- phosphanilydene) -ethanone is added, then the reaction mixture is left to stay overnight. The given solution is extracted with 2X100 ml of water, dried over magnesium sulphate, filtrated and the solvent is removed by vacuum distillation. 400 ml of diethyl ether is added to the obtained 380 g oily product and the mixture is stirred for 10 minutes. The precipitated crystalline triphenylphosphin oxide is filtered, and washed with 3X100 ml diethyl ether. The filtrate is evaporated in vacuum. The residue is 300 g. Then 100 g of isopropanol is added to the residue at room temperature. The reaction mixture is stirred for 10 minutes, filtered, the crystalline is washed with 2x 20 ml isopropanol. Thus 87,9g (54,1%) titled product is obtained. Mp.: 80, 5-82°C. The filtrate is evaporated to 200 ml, is left to stay overnight in fridge, then the precipitated crystalline is filtered, washed with 2X20 ml cold (5OC) isopropanol. Thus 50,1 g (30,8%) second product is obtained. Mp. : 80- 82°C. Both products are ready to use in the subsequent reaction. The total yield is 84,9% the optical rotation of product is [A] D20 (C=1, CHC13) = + 28, 1°.
References:
WO2004/41805,2004,A1 Location in patent:Page 45-46