
7H-Pyrrolo[1,2-c]imidazol-7-one,5,6-dihydro-(9CI) synthesis
- Product Name:7H-Pyrrolo[1,2-c]imidazol-7-one,5,6-dihydro-(9CI)
- CAS Number:426219-43-4
- Molecular formula:C6H6N2O
- Molecular Weight:122.12
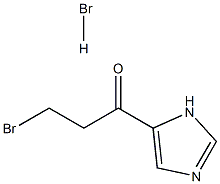
1346155-45-0
1 suppliers
inquiry
![7H-Pyrrolo[1,2-c]imidazol-7-one,5,6-dihydro-(9CI)](/CAS/20150408/GIF/426219-43-4.gif)
426219-43-4
43 suppliers
$65.00/10mg
Yield:426219-43-4 61%
Reaction Conditions:
with triethylamine in acetonitrile at 70; for 2.5 h;
Steps:
4.5. 5,6-Dihydro-7H-pyrrolo[1,2-c]imidazol-7-one (6)
A solution of Et3N (15.3 mL, 110 mmol) in CH3CN (25 mL) was added dropwise to a hot (70 °C) suspension of 5 (28.5 g, 100 mmol) in CH3CN (1100 mL) and the mixture was stirred at 70 °C for 2 h. Triethylamine (25 mL, 179 mmol) was then added to the mixture, and the solution was stirred at 70 °C for a further 30 min. After cooling to room temperature, the insoluble material was filtered off and the filtrate was concentrated under reduced pressure. The residue was dissolved in ethyl acetate and the insoluble material was again filtered off. The filtrate was concentrated under reduced pressure and the resulting residue was separated by chromatography on silica gel (CH2Cl2/5% ammonia in MeOH = 10:1) to give 6 (6.67 g, 61%) as a colorless powder. 1H NMR(CDCl3) δ: 3.24 (2H, t, J = 6.5 Hz), 4.41 (2H, t, J = 6.5 Hz), 7.60 (1H, s), 7.74 (1H, s). IR(KBr): 3121, 1713, 1537, 1489, 1412, 1319, 1204, 1109 cm-1.
References:
Ruchelman, Alexander L.;Man, Hon-Wah;Chen, Roger;Liu, Wei;Lu, Ling;Cedzik, Dorota;Zhang, Ling;Leisten, Jim;Collette, Alice;Narla, Rama Krishna;Raymon, Heather K.;Muller, George W. [Bioorganic and Medicinal Chemistry,2011,vol. 19,# 21,p. 6356 - 6374] Location in patent:experimental part
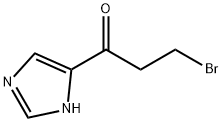
1346218-36-7
6 suppliers
inquiry
![7H-Pyrrolo[1,2-c]imidazol-7-one,5,6-dihydro-(9CI)](/CAS/20150408/GIF/426219-43-4.gif)
426219-43-4
43 suppliers
$65.00/10mg
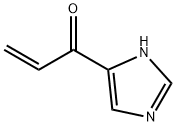
57531-35-8
0 suppliers
inquiry
![7H-Pyrrolo[1,2-c]imidazol-7-one,5,6-dihydro-(9CI)](/CAS/20150408/GIF/426219-43-4.gif)
426219-43-4
43 suppliers
$65.00/10mg