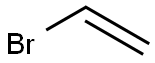
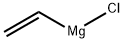(7R,11R)-3,7,11,15-tetramethylhexadec-1-en-3-ol synthesis
- Product Name:(7R,11R)-3,7,11,15-tetramethylhexadec-1-en-3-ol
- CAS Number:395645-30-4
- Molecular formula:C20H40O
- Molecular Weight:296.531
Yield:395645-30-4 96%
Reaction Conditions:
in tetrahydrofuran at 20 - 30; for 1.41 h;Inert atmosphere;
Steps:
Experiment E6-lll:Vinylation of (6R,10R)-6,10,14-trimethylpentadecan-2-one (step h') A dried 100 ml. four-necked flask equipped with overhead stirrer, thermometer, condenser and argon inlet was evacuated and purged with argon. Vinylmagnesium chloride (18.3 mL of a 1.6 M solution in THF, 29.0 mmol, 1.59 eq.) was added at room temperature. A solution of (6R,10R)-6,10,14-trimethylpentadecan-2-one (5.00 g, 18.3 mmol, 98.2%, 1.0 eq.) in dry THF (20 mL) was added slowly within 25 min. The exothermic reaction was maintained between 25 and 30 °C internal temperature by cooling with an ice bath. After complete addition the reaction was allowed to stir at room temperature for 1 h. Saturated NH4Cl solution (10 mL) was added carefully to quench excess Grignard reagent. Pentane (150 mL), water (150 mL) and brine (150 mL) was added. The organic phase was extracted with brine (2 x 150 mL) and the aqueous phase was back-extracted with pentane (2 x 150 mL). The combined organic phases were dried (MgSO4) and concentrated in vacuo, resulting in a colorless oil (5.58 g). The crude product was purified by vacuum distillation in a Kugelrohr apparatus. The main fraction distilled at 143 °C/3.5x10 2 mbar, furnishing (R,R)-isophytol (=(7R,11R)-3,7,11,15-tetramethylhexadec-1-en-3-ol) as colorless oil with a purity of 99.3% (5.271 g, 96% yield).
References:
WO2014/96063,2014,A1 Location in patent:Page/Page column 61

1614254-33-9
0 suppliers
inquiry

395645-30-4
5 suppliers
inquiry
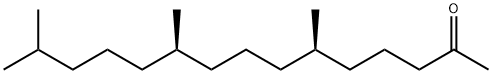
16825-16-4
9 suppliers
inquiry

395645-30-4
5 suppliers
inquiry

1604-35-9
33 suppliers
inquiry

395645-30-4
5 suppliers
inquiry