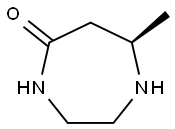
(7R)-HEXAHYDRO-7-METHYL-5-H-1,4-DIAZEPIN-5-ONE synthesis
- Product Name:(7R)-HEXAHYDRO-7-METHYL-5-H-1,4-DIAZEPIN-5-ONE
- CAS Number:1394957-73-3
- Molecular formula:C6H12N2O
- Molecular Weight:128.17

90673-37-3
30 suppliers
$117.00/50mg

1394957-73-3
5 suppliers
inquiry
Yield:95.122 % ee
Reaction Conditions:
with (2S,3S)-di-4-toluoyltartaric acid in methanol;stereoselective reaction;
Steps:
Chiral Resolution of 7-methyl-l,4-diazepan-5-one:
Chiral resolutions were achieved with several chiral acids with varying degree of induction by performing a systematic screening. Here, the best hit will be described as a representative example. LTTA corresponds to the enantiomer (+)-Di-0,0'-toluyl-L-tartaric acid. A solution of the chiral acid (62.8 mg, 0.16 mmol) in MeOH (0.5 mL) and a solution of ra- cemic 7-methyl-l,4-diazepan-5-one (41.4 mg, 0.32 mmol) in MeOH (0.8 mL) were combined and allowed to crystallize over night. The solid was filtered and the enantiomeric excess was determined by HPLC. The enantiomeric ratio was > 40 : 1 (R : S) wherein, the absolute stereochemistry is determined according to literature proceedings.
References:
WO2016/20404,2016,A1 Location in patent:Page/Page column 75