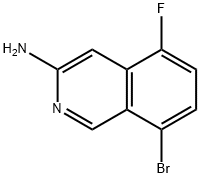
8-bromo-5-fluoroisoquinolin-3-amine synthesis
- Product Name:8-bromo-5-fluoroisoquinolin-3-amine
- CAS Number:1221974-48-6
- Molecular formula:C9H6BrFN2
- Molecular Weight:241.06
![Ethanimidamide, N-[(2-bromo-5-fluorophenyl)methyl]-2,2-diethoxy-](/CAS/20210305/GIF/1221974-47-5.gif)
1221974-47-5
0 suppliers
inquiry

1221974-48-6
12 suppliers
inquiry
Yield:1221974-48-6 40.6%
Reaction Conditions:
with sulfuric acid in dichloromethane at 80; for 16 h;
Steps:
346.D Step D: 8-bromo-5-fluoroisoquinolin-3-amine
To a mixture of N-(2-bromo-5-fluorobenzyl)-2,2-diethoxyacetimidamide(1.6 g, 4.80 mmol) and dichloromethane (2 mL) was added H2SO4 (1.280 ml., 24.01mmol). The reaction mixture was stirred at 80° C. for 16 hours, cooled to rt., diluted with EtOAc (100 ml.), quenchedwith ice-water, and neutralized with NaHC03 solution. Theorganic phase was washed with water, dried over Na2S04 ,and evaporated. The residue was purified on an 80 g Thompsonsilica cartridge (3% to 100% EtOAc in Hexanes, 1200mL). The desired product was obtained as a pale yellow solid(0.47 g, 1.950 mmol, 40.6% yield).
References:
JP5714745,2015,B2 Location in patent:Paragraph 0715; 0719
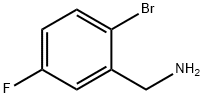
747392-34-3
87 suppliers
$12.00/1g

1221974-48-6
12 suppliers
inquiry
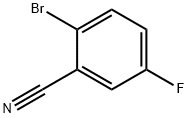
57381-39-2
202 suppliers
$9.00/5g

1221974-48-6
12 suppliers
inquiry