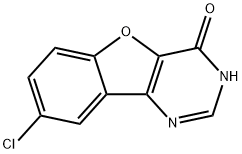
8-Chloro[1]benzofuro[3,2-d]pyrimidin-4(3H)-one synthesis
- Product Name:8-Chloro[1]benzofuro[3,2-d]pyrimidin-4(3H)-one
- CAS Number:328277-09-4
- Molecular formula:C10H5ClN2O2
- Molecular Weight:220.61
329210-07-3
24 suppliers
$70.00/250mg
122-51-0
488 suppliers
$10.00/5ml
![8-Chloro[1]benzofuro[3,2-d]pyrimidin-4(3H)-one](/CAS/GIF/328277-09-4.gif)
328277-09-4
6 suppliers
inquiry
Yield:-
Reaction Conditions:
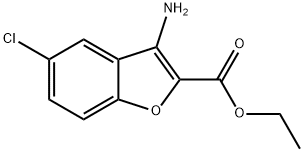
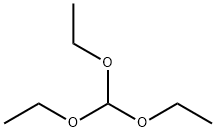
Stage #1: 3-amino-5-chloro-benzofuran-2-carboxylic acid ethyl ester;orthoformic acid triethyl ester at 200; for 0.166667 h;Microwave irradiation;
Stage #2: with ammonia in methanol at 140; for 0.166667 h;Microwave irradiation;
Steps:
2.G
Intermediate G: 8-Chloro-3H-benzo[4,5]furo[3,2-d]pyrimidin-4-one [Show Image] A suspension of ethyl 3-amino-5-chlorobenzofuran-2-carboxylate (intermediate F, 0.3g) in triethyl orthoformate (2mL) is irradiated in a microwave at 200°C for 10 minutes. The resultant yellow solution is evaporated to dryness and the residue was dissolved in a solution of ammonia in methanol (2M, 2mL). The mixture is carefully irradiated in a microwave at 140°C for 10 minutes and the resultant precipitate is collected by filtration and washed with diethyl ether. The filtrate is evaporated to dryness, the residue is triturated with acetonitrile and the solid was collected by filtration. The solids are combined to give 8-chloro-3H-benzo[4,5]furo[3,2-d]pynnudin-4-one (0.175 g) as a grey powder. 1H NMR (DMSO-D6): δ 7.6 (d, 1H), 7.9 (d, 1H), 8.1 (s, 1H). 8.25 (s, 1H).
References:
EP1767537,2007,A1 Location in patent:Page/Page column 12-13

329210-07-3
24 suppliers
$70.00/250mg
![8-Chloro[1]benzofuro[3,2-d]pyrimidin-4(3H)-one](/CAS/GIF/328277-09-4.gif)
328277-09-4
6 suppliers
inquiry