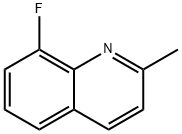
8-Fluoro-2-Methyl-Quinoline synthesis
- Product Name:8-Fluoro-2-Methyl-Quinoline
- CAS Number:46001-36-9
- Molecular formula:C10H8FN
- Molecular Weight:161.18
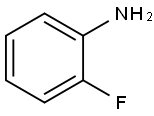
348-54-9
413 suppliers
$10.00/1g

123-73-9
195 suppliers
$40.00/5g

46001-36-9
36 suppliers
$90.00/50mg
Yield:46001-36-9 77%
Reaction Conditions:
Stage #1: 2-Fluoroaniline;crotonaldehydewith hydrogenchloride in water;toluene at 100; for 2 h;Inert atmosphere;
Stage #2: with sodium hydroxide in water;
Steps:
2
Intermediate 2 8-Fluoro-2-methylquinoline 2-Fluoroaniline (commercially available, for example, from Aldrich) (2.2 ml, 22.5 mmol) was dissolved in 5M aqueous hydrochloric acid (100 ml), then toluene (30 ml) and crotonaldehyde (3.71 ml, 45mmol) were added. The reaction was heated at 1000C for 2 h under nitrogen with vigorous stirring. The reaction mixture was left to cool, and the aqueous layer separated. This was neutralised using aqueous sodium hydroxide and extracted with dichloromethane (2 x 50 ml). The organic solutions were combined, dried over a ptfe frit and evaporated to leave a brown gel. This was purified by Flashmaster Il (100 g silica SPE, loaded in dichloromethane, eluted with 0-50% ethyl acetate-cyclohexane over 1 h). The solvent was evaporated to give the title compound (2.8 g,77%). LCMS RT=2.19 min, ES+ve m/z 162 (M+H)+.
References:
WO2010/94643,2010,A1 Location in patent:Page/Page column 42

123-73-9
195 suppliers
$40.00/5g

348-54-9
413 suppliers
$10.00/1g

46001-36-9
36 suppliers
$90.00/50mg
66023-21-0
17 suppliers
inquiry

348-54-9
413 suppliers
$10.00/1g

46001-36-9
36 suppliers
$90.00/50mg

348-54-9
413 suppliers
$10.00/1g

46001-36-9
36 suppliers
$90.00/50mg