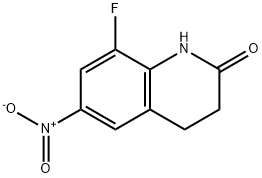
8-FLUORO-6-NITRO-3,4-DIHYDROQUINOLIN-2(1H)-ONE synthesis
- Product Name:8-FLUORO-6-NITRO-3,4-DIHYDROQUINOLIN-2(1H)-ONE
- CAS Number:590422-02-9
- Molecular formula:C9H7FN2O3
- Molecular Weight:210.1619
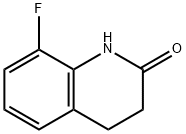
143268-79-5
48 suppliers
$45.00/10mg

590422-02-9
12 suppliers
inquiry
Yield:590422-02-9 92%
Reaction Conditions:
with sulfuric acid;nitric acid in water at -5; for 0.333333 - 0.666667 h;Product distribution / selectivity;
Steps:
52; 55
Example 52 8-Fluoro-6-nitro-3,4-dihydroquinolin-2(1H)-one To a stirred solution of 8-fluoro-3,4-dihydroquinolin-2(1H)-one (see Example 41 for details) (2 g, 12.11 mmol) in sulfuric acid (12 ml), cooled to -5° C. was added nitric acid, 90% (0.565 ml, 12.11 mmol) as a 1:1 solution in water. The reaction mixture was stirred at -5° C. for 20 min, then quenched via addition of ice, precipitating the product which was collected by filtration. The pale solid was then dissolved in dichloromethane, dried, filtered and concentrated, and then chromatographed on silica gel using 0-10% ethyl acetate in dichloromethane as eluent to give the desired 8-fluoro-6-nitro-3,4-dihydroquinolin-2(1H)-one (2.33 g, 11.09 mmol, 92% yield). 1H NMR (DMSO-d6) δ 10.73 (brs, 1H), 8.03 (m, 1H), 8.02 (m, 1H), 3.08 (t, J=7.5 Hz, 2H), 2.57 (t, J=7.5 Hz, 1H). 8-Fluoro-6-nitro-3,4-dihydroquinolin-2(1H)-one; To a stirred solution of 8-fluoro-3,4-dihydroquinolin-2(1H)-one (see Example 41 for details), (5 g, 30.3 mmol) in sulfuric acid, cooled to -5° C. was added nitric acid, fuming (1.413 ml, 30.3 mmol) as a 1:1 mixture in water. The resulting mixture was stirred at -5° C. for 20 min. After 20 min the reaction was quenched via addition of ice, precipitating the product which was collected by filtration. The filter cake was dissolved in dichloromethane and a minimal amount of methanol, dried, filtered and concentrated onto silica gel then chromatographed using 0-10% ethyl acetate in dichloromethane to give the desired 8-fluoro-6-nitro-3,4-dihydroquinolin-2(1H)-one (5.9 g, 28.1 mmol, 93% yield). 1H NMR (DMSO-d6) δ 10.73 (brs, 1H), 8.03 (m, 1H), 8.02 (m, 1H), 3.08 (t, J=7.5 Hz, 2H), 2.57 (t, J=7.5 Hz, 1H).
References:
US2008/234237,2008,A1 Location in patent:Page/Page column 87; 91
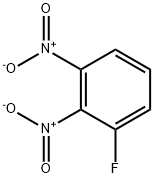
361-12-6
0 suppliers
inquiry

590422-02-9
12 suppliers
inquiry