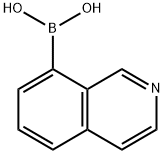
8-isoquinolinyl-boronic acid synthesis
- Product Name:8-isoquinolinyl-boronic acid
- CAS Number:721401-43-0
- Molecular formula:C9H8BNO2
- Molecular Weight:172.98
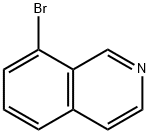
63927-22-0
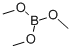
121-43-7

721401-43-0
To a solution of 8-bromoisoquinoline (2.0 g, 9.6 mmol) in tetrahydrofuran (THF, 40 mL) was slowly added n-butyllithium (n-BuLi, 2.5 M, 4.2 mL, 10.6 mmol) dropwise at -78 °C and under nitrogen protection. After 1 hour of reaction, trimethyl borate (B(OMe)3, 2.0 g, 19.2 mmol) was added, followed by slow warming of the reaction mixture to 0 °C and kept for 1 hour. Upon completion of the reaction, the reaction was quenched with saturated aqueous sodium bicarbonate (NaHCO3) followed by three extractions with ethyl acetate (EtOAc). The organic layers were combined, washed with saturated saline, dried over anhydrous sodium sulfate (Na2SO4) and concentrated under reduced pressure. The residue was purified by silica gel column chromatography to afford the target product 8-isoquinolineboronic acid (680 mg, 41% yield) as a light yellow solid. Liquid chromatography-mass spectrometry (LCMS) analysis showed [M+H]+ m/z 174.1.

63927-22-0
264 suppliers
$7.00/250mg
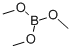
121-43-7
382 suppliers
$13.00/25mL

721401-43-0
96 suppliers
$45.00/25mg
Yield: 41%
Reaction Conditions:
Stage #1:8-bromo-isoquinoline with n-butyllithium in tetrahydrofuran at -78; for 1 h;Inert atmosphere;
Stage #2:Trimethyl borate in tetrahydrofuran at 0; for 1 h;
Steps:
27 Example 27. Synthesis of 3-(isoq u inol i n-8-yloxy)-4-methyl-N-(3-(4-methyl-H-imidazol-1 -yl)-5-(4-methylpiperazin-1 -yl)phenyl)benzamide.
To a solution of 8-bromoisoquinoline (2.0 g, 9.6 mmol) in THE (40 mL) was added dropwise n-BuLi (2.5 M, 4.2 mL, 10.6 mmol) at -78 °C under nitrogen. After 1 hour, B(OMe)3 (2.0 g, 19.2 mmol) was added to the reaction and the mixture was warmed to 0 °C for 1 hour. The reaction was quenched by aqueous NaHCO3 andextracted with EtOAc 3 times. The combined organic layers were washed with brine, dried over Na2SO4 and concentrated. The residue was purified by silica gel column chromatography to give 75 (680 mg, 41%) as a slightly yellow solid. LCMS (m/z: m+1):174.1.
References:
CARDIO THERAPEUTICS PTY LTD;TREUTLEIN, Herbert;ZENG, Jun;DIXON, Ian;JAMES, Ian;PALMER, James T WO2018/165718, 2018, A1 Location in patent:Page/Page column 93

63927-22-0
264 suppliers
$7.00/250mg

721401-43-0
96 suppliers
$45.00/25mg