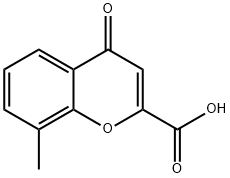
8-METHYL-4-OXO-4H-CHROMENE-2-CARBOXYLIC ACID synthesis
- Product Name:8-METHYL-4-OXO-4H-CHROMENE-2-CARBOXYLIC ACID
- CAS Number:38243-78-6
- Molecular formula:C11H8O4
- Molecular Weight:204.18
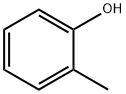
95-48-7
433 suppliers
$18.00/25g

762-42-5
356 suppliers
$8.00/10g

38243-78-6
13 suppliers
$110.00/50mg
Yield:38243-78-6 32%
Reaction Conditions:
Stage #1: ortho-cresol;dimethyl acetylenedicarboxylatewith lithium hydroxide monohydrate in dichloromethane at 20; for 2 h;
Stage #2: with lithium hydroxide monohydrate in tetrahydrofuran;water at 20; for 18 h;
Steps:
Prep.43. Ethyl 8-methyl-4-oxo-4-chromene-2-carboxylate.
A mixture of hydroxylithium hydrate (3151 mg, 75.09 mmol) in DCM (50 mL) was prepared at rt and dimethyl but-2-ynedioate (2.31 mL, 2668 mg, 18.77 mmol) was added followed by o-cresol (2-methyIpheno) (2.0 mL, 2030 mg, 18.77 mmol) and the mixture stirred at rt for 2h. The mixture was then washed with water (2 x 20 mL), filtered through a phase separator and the crude filtrate concentrated under reduced pressure to afford a green oil. The oil was dissolved in THF (25 mL) and hydroxylithium hydrate (3151 mg, 75.09 mmol) in water (25 mL) added and the mixture stirred at rt for 18h. The mixture was then concentrated under reduced pressure then dissolved in water (20 mL) and 6M aqueous HCI was added and the solution adjusted to pH 1-2 and then the mixture was stirred at rt for 1 h. Resulting precipitate was filtered, washed with water (2 x 50 mL) and dried under vacuum. The crude precipitate was then suspended in concentrated sulphuric acid (30 mL) and heated to 80°C for 18h and cooled to rt. Mixture was cooled to 0°C and water (40 mL) added to mixture (light brown precipitate formed) and stirred for 30 mins then allowed to warm to rt and stirred for a further 30 mins. Precipitate then filtered through a fritted column and crude solid re-suspended in water (60 mL) and stirred for 2 hours then filtered, filter cake washed with water (2 x 20 mL) and precipitate dried under vacuum. Solid suspended in acetonitrile and concentrated under reduced pressure to afford 8-methyl-4-oxo-chromene-2-carboxylic acid (1318 mg, 32 %) as a tan coloured solid. 1H NMR (500 MHz, DMSO) δ 7.90 (d, J=7.8 Hz, 1 H), 7.75 (d, J=7.0 Hz, 1 H), 7.44 (t, J=7.6 Hz, 1 H), 6.92 (s, 1 H), 2.52 - 2.50 (m, 3H) (OH proton not observed); LCMS m/z 202 (M-H)-.
References:
WO2017/221002,2017,A1 Location in patent:Page/Page column 159