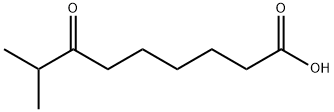
8-METHYL-7-OXONONANOIC ACID synthesis
- Product Name:8-METHYL-7-OXONONANOIC ACID
- CAS Number:55277-54-8
- Molecular formula:C10H18O3
- Molecular Weight:186.25
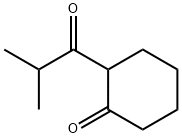
39207-65-3
108 suppliers
$24.00/1g

55277-54-8
10 suppliers
$303.00/1g
Yield:-
Reaction Conditions:
with potassium hydroxide in water; for 1 h;Reflux;
Steps:
1
The obtained crude product was added to a lye containing 61.2 g of potassium hydroxide in 88.5 g of water, and reacted under reflux for 1 hour. After cooling, it was adjusted to neutrality with dilute hydrochloric acid, and the organic layer was separated into the crude carbonyl carboxylic acid compound. Add 248.9g of diethylene glycol and 3.0 equivalents of potassium hydroxide into the reaction flask. After stirring until the potassium hydroxide is dissolved, add the obtained crude carbonyl carboxylic acid compound and 45.1g of hydrazine hydrate. The temperature is raised to 145°C and reacted for 1 hour. Water was distilled under reduced pressure. After the temperature in the reaction system reached 210°C, the temperature was kept for 3 hours. Diethylene glycol was distilled under reduced pressure. After the evaporation, water was added to dissolve the residue. After the impurities were extracted with ethyl acetate 3 times, the aqueous solution was adjusted to acidity. The product was extracted with ethyl acetate, dried over anhydrous sodium sulfate, and distilled under reduced pressure to obtain 42.3 g of pure 8-methylnonanoic acid.
References:
CN112920042,2021,A Location in patent:Paragraph 0031; 0045; 0049
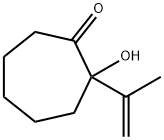
55277-45-7
0 suppliers
inquiry

55277-54-8
10 suppliers
$303.00/1g
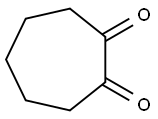
3008-39-7
12 suppliers
inquiry

55277-54-8
10 suppliers
$303.00/1g