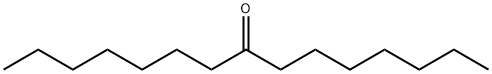
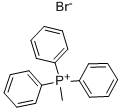
8-Methylenepentadecane synthesis
- Product Name:8-Methylenepentadecane
- CAS Number:55668-09-2
- Molecular formula:C16H32
- Molecular Weight:224.43
Yield:55668-09-2 66%
Reaction Conditions:
Stage #1: Methyltriphenylphosphonium bromidewith potassium tert-butylate in toluene at 20; for 1 h;Inert atmosphere;Reflux;
Stage #2: 8-Pentadecanon in toluene at 20; for 0.5 h;Inert atmosphere;
Steps:
For the synthesis of 2-heptyl-1-nonene10
t-BuOK (0.6688 g, 6.0 mmol) was added in four portions to a solution of methyltriphenylphosphonium bromide (1.8048 g, 5.1 mmol) in dry toluene (38 mL) under nitrogen at room temperature. The reaction mixture was heated at reflux for 1 h and then cooled to room temperature. 8-Pentadecanone (1.1294 g, 5.0 mmol) in dry toluene was added dropwise and stirred for 30 min. The reaction mixture was quenched with sat. NH4Cl. The organic layer was separated and the aqueous layer was extracted with Et2O. The combined organic layer was washed with brine, dried over MgSO4, and filtered. The filtrate was concentrated in vacuo. The resulting residue was purified by column chromatography on silica gel (hexane) to afford 2-heptyl-1-nonene. Colorless liquid (0.7423 g, 66% yield). 1H NMR (500 MHz, CDCl3) δ 4.71 (s, 2H), 2.02 (t, J = 7.7 Hz, 4H), 1.45 (d, J = 6.6 Hz, 4H), 1.31 (m, 16H), 0.98-0.87 (m, 6H).
References:
Tanaka, Kenta;Kishimoto, Mami;Hoshino, Yujiro;Honda, Kiyoshi [Tetrahedron Letters,2018,vol. 59,# 19,p. 1841 - 1845] Location in patent:supporting information

124-13-0
356 suppliers
$7.00/25g

55668-09-2
0 suppliers
inquiry
1653-35-6
33 suppliers
inquiry

55668-09-2
0 suppliers
inquiry

13125-66-1
108 suppliers
$60.00/5g

55668-09-2
0 suppliers
inquiry