
8-Quinolinol, 7-bromo-5-nitro- synthesis
- Product Name:8-Quinolinol, 7-bromo-5-nitro-
- CAS Number:23521-17-7
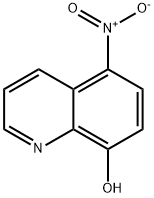
- Molecular formula:C9H5BrN2O3
- Molecular Weight:269.05
4008-48-4
283 suppliers
$5.00/100mg

23521-17-7
3 suppliers
inquiry
Yield:23521-17-7 65%
Reaction Conditions:
with N-Bromosuccinimide;sulfuric acid in tetrahydrofuran at 20; for 1.5 h;
Steps:
[00258] 7-Bromo-8-hydroxy-4-nitroquinoline (16).
N-bromosuccinimide (492 mg, 2.76 mmol) was added to a stirring solution of nitroxoline 17 (500 mg, 2.63 mmol) in tetrahydrofuran (16 mL), followed by the addition of a catalytic amount of concentrated sulfuric acid (15 The resulting mixture was then allowed to stir for 90 minutes at room temperature. At that time, the reaction mixture was poured onto 50 mL of water and the resulting suspension was filtered. The solid was then collected and dried under vacuum to afford 16 as a bright orange solid (457 mg, 65% yield). Note: This protocol was previously described by Boger and coworkers. The spectral data and melting point that we obtained for this compound are in complete agreement with data previously reported (D. L. Boger, S. R. Duff, J. S. Panek, M. Yasuda, J. Org. Chem. 1985, 50, 5782-5789). 1H NMR (400 MHz, d6- DMSO): δ 9.32 (d, J = 8.8 Hz, 1H), 8.97 (d, J = 4.4 Hz, 1H), 8.73 (s, 1H), 7.98 (dd, / = 8.8, 4.4 Hz, 1H). 13C NMR (100 MHz, d6-DMSO): δ 159.9, 147.3, 135.7, 135.3, 132.4, 131.5, 125.5, 122.8, 104.5. MP: 198-200 °C, lit. 199 °C (D. L. Boger, S. R. Duff, J. S. Panek, M. Yasuda, J. Org. Chem. 1985, 50, 5782-5789).
References:
WO2016/154051,2016,A1 Location in patent:Paragraph 00258

99-57-0
9 suppliers
$22.00/25g

23521-17-7
3 suppliers
inquiry
3565-26-2
89 suppliers
$16.00/1g

23521-17-7
3 suppliers
inquiry