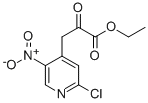
ETHYL 3-(2-CHLORO-5-NITROPYRIDIN-4-YL)-2-OXOPROPANOATE synthesis
- Product Name:ETHYL 3-(2-CHLORO-5-NITROPYRIDIN-4-YL)-2-OXOPROPANOATE
- CAS Number:800401-66-5
- Molecular formula:C10H9ClN2O5
- Molecular Weight:272.64
Yield:800401-66-5 92%
Reaction Conditions:
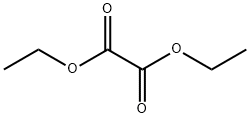
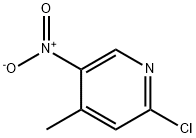
Stage #1: 2-chloro-4-methyl-5-nitro-pyridine;oxalic acid diethyl esterwith 1,8-diazabicyclo[5.4.0]undec-7-ene at 20;Inert atmosphere;
Stage #2: with hydrogenchloride in lithium hydroxide monohydrate;
Steps:
1a.1
Step 1: synthesis of ethyl 3-(2-chloro-5-nitropyridin-4-yl)-2-oxopropanoate 10-aTo a solution of 2-Chloro-4-methyl-5-nitropyridine (20 g, 115 mmoles) in diethyl oxalate (150 mL) under a nitrogen atmosphere was added DBU (20 mL, 1.15 eq) dropwise and stirring at room temperature was continued overnight. The reaction mixture was then poured into 200 mL ice water and this mixture was acidified with 140 mL IN HCl solution. The semi solids were allowed to fall out and the solvent on top of it was decanted off. The residue was then stirred in ice cold ethanol. The precipitate was filtered off and dried in vacuo to give 29.04 g (92% yield) of the targeted compound 10-a. m/z = 273 (M+H)+; 1H NMR (400 MHz, DMS0 ) ppm 1.30 (t, J = 7.0 Hz, 3H), 4.32 (q, J = 7.1 Hz, 2H), 4.68 (s, 2H), 6.68 (s, lH), 7.80 (s, lH), 8.25 (s, lH), 9.00 (s,lH), 9.14 (s, lH).
References:
WO2012/80450,2012,A1 Location in patent:Page/Page column 23-24
23056-33-9
328 suppliers
$3.00/1g

800401-66-5
20 suppliers
inquiry