
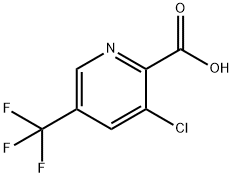
3-Chloro-2-(chlorocarbonyl)-5-(trifluoromethyl)pyridine, 3-Chloro-5-(trifluoromethyl)picolinoyl chloride synthesis
- Product Name:3-Chloro-2-(chlorocarbonyl)-5-(trifluoromethyl)pyridine, 3-Chloro-5-(trifluoromethyl)picolinoyl chloride
- CAS Number:80194-72-5
- Molecular formula:C7H2Cl2F3NO
- Molecular Weight:244
80194-68-9
114 suppliers
$25.00/1g

80194-72-5
13 suppliers
$102.50/250mgs:
Yield:80194-72-5 100%
Reaction Conditions:
with oxalyl dichloride;N,N-dimethyl-formamide in dichloromethane;toluene at 0 - 20; for 2 h;
Steps:
1
3-Chloro-5-(trifluoromethyl)picolinic acid (72.3 mg, 320 μπιο) was suspended in dichloromethane (5 mL), the suspension was cooled to 0-5°C (ice bath) and oxalyl chloride (56.9 mg, 39.3 μ, 448 μιηο) as well as dimethylformamide (0.308 M in toluene, 51.9 μ, 16 μιηο) were added. The mixture was stirred for 2 h at room temperature. Then, it was concentrated in vacuo (40°C, 5 mbar) and dried azeotropically by two cycles of addition of toluene (3 mL) followed by concentration in vacuo to afford 3-chloro-5-(trifluoromethyl)picolinoyl chloride as yellow oil (78 mg, quant.). After that, tert-butyl ((3aS,4R,8R)-4-(5-amino-2-fluorophenyl)-4,7,7- trimethyl-8-oxido-3,3a,4,7-tetrahydro-2H-isothiazolo[l,5-a] [l,4]thiazin-6-yl)carbamate (Int- 16ABp, 80 mg, 188 μιηο) was dissolved in dichloromethane (5 mL), the solution cooled to 10°C and N,N-diisopropylethylamine (36.5 mg, 49.4 μ, 283 μιηο) was added, followed by a solution of 3-chloro-5-(trifluoromethyl)picolinoyl chloride (vide supra, 62 mg, 256 μιηο) in dichloro- methane (4 mL). The reaction mixture was stirred for 15 min at 10°C. Then, methanol (2 mL) was added, the mixture was stirred for 5 min at room temperature and concentrated in vacuo. The crude was purified by column chromatography (silica gel, 12 g, eluting with ethyl acetate / n-heptane, gradient 25:75 to 100:0) to yield, after drying in vacuo (40°C, 5 mbar), the title compound as an off-white solid (100 mg, 84% yield). HPLC (method LCMS_fglm) tR = 1.35 min. MS (ES+) m/z 632.5 [M+H] .
References:
WO2017/25491,2017,A1 Location in patent:Page/Page column 56; 57

80194-68-9
114 suppliers
$25.00/1g

79-37-8
463 suppliers
$17.67/10gm:

80194-72-5
13 suppliers
$102.50/250mgs: