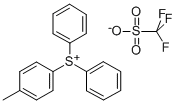
(4-METHYLPHENYL)DIPHENYL SULFONIUM TRIFLUOROMETHANESULFONATE synthesis
- Product Name:(4-METHYLPHENYL)DIPHENYL SULFONIUM TRIFLUOROMETHANESULFONATE
- CAS Number:81416-37-7
- Molecular formula:C20H17F3O3S2
- Molecular Weight:426.47
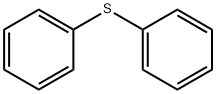
139-66-2
283 suppliers
$5.00/10g

1204518-02-4
34 suppliers
$28.00/100MG

81416-37-7
35 suppliers
$90.00/1g
Yield:81416-37-7 96%
Reaction Conditions:
with Cu(OAc)2*H2O in 1,2-dichloro-ethane at 100; for 15 h;Catalytic behavior;Sealed tube;Reagent/catalyst;Temperature;Time;
Steps:
Procedure for sulfonium salt synthesis by aryl-group transfer using mesityliodonium(III) salt 2.
General procedure: Diarylsulfide 1(0.50 mmol), aryl(mesityl)iodonium(III) salt 2 (0.60 mmol, 1.2 equiv.) and Cu(OAc)2·H2O (9.9 mg, 0.05 mmol, 10mol % relative to diarylsulfide 1) were dissolved in 1,2-dichloroethane (DCE, 5 mL) in a sealed tube. The reaction mixture was stirred at the indicated temperature and time (see Table 1). It was then cooled to room temperature, and the resulting solution was purified by column chromatography on silica gel (dichloromethane/acetone) to give triarylsulfonium triflate 3. Sulfonium salts are generally light sensitive, and coloration of the compounds would occur during the column chromatography and evaporation. (4-Methylphenyl)diphenylsulfonium triflate (3aa).27 Gray solid; mp 97-98 °C. IR: 3064, 2928, 1591, 1476 cm-1. 1H NMR: δ 2.46 (s, 3H, CH3), 7.49 (d, J 8.3 Hz, 2H, ArH), 7.61 (d, J 8.8 Hz, 2H, ArH), 7.68-7.76 (m, 10H, ArH) ppm. 13C NMR: δ 21.0, 120.7 (q, JCF 320.0 Hz, OTf), 121.4, 125.5, 131.1, 131.3, 131.4, 131.9, 134.2, 145.5 ppm.
References:
Dohi, Toshifumi;Hayashi, Takumi;Kumar, Ravi;Miyamoto, Naoki;Nojiri, Haruna;Takenaga, Naoko;Yoto, Yusuke [Arkivoc,2021,vol. 2021,# 7,p. 7 - 18]

945-51-7
247 suppliers
$5.00/5g

81416-37-7
35 suppliers
$90.00/1g
3699-01-2
81 suppliers
$45.00/250mg
66003-76-7
139 suppliers
$6.00/250mg

81416-37-7
35 suppliers
$90.00/1g
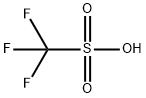
1493-13-6
608 suppliers
$12.00/5g

945-51-7
247 suppliers
$5.00/5g
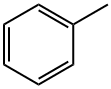
108-88-3
7 suppliers
$16.10/100ml

81416-37-7
35 suppliers
$90.00/1g

27607-77-8
488 suppliers
$10.00/5g

945-51-7
247 suppliers
$5.00/5g
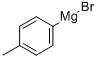
4294-57-9
123 suppliers
$50.00/10ml

81416-37-7
35 suppliers
$90.00/1g