
(22R,23R)-2A,3A,22,23-TETRAHYDROXY-24-ETHYL-BETA-HOMO-7-OXA-5A-CHOLESTAN-6-ONE synthesis
- Product Name:(22R,23R)-2A,3A,22,23-TETRAHYDROXY-24-ETHYL-BETA-HOMO-7-OXA-5A-CHOLESTAN-6-ONE
- CAS Number:82373-95-3
- Molecular formula:C29H50O6
- Molecular Weight:494.71
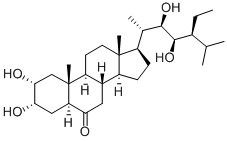
83509-42-6
11 suppliers
$140.00/25mg

82373-95-3
46 suppliers
inquiry
Yield:82373-95-3 95%
Reaction Conditions:
with sodium hypochlorite in water at 25; for 0.5 h;Ionic liquid;Baeyer-Villiger Ketone Oxidation;Reagent/catalyst;Temperature;
Steps:
1-5
Successively add intermediate b (392.1g), [MIM-CH2CHClCOOH]2HPW12O40 (78.3g), water (784ml) to the reactor. Add sodium hypochlorite (95.5g) under stirring at 25 ° C. The reaction is held for 0.5h. Using equal volumes of saturated sodium thiosulfate solution wash three times, extracted with ethyl acetate, and centrifuged to recover [MIM-CH2CHClCOOH]2HPW12O40, washed with saturated brine, and dried to obtain 28-homobrassinolide (400.5 g, purity 96.1%, yield 95.0%).
References:
CN110452284,2019,A Location in patent:Paragraph 0012-0013; 0024; 0026-0027; 0029-0030; 0036; 0038
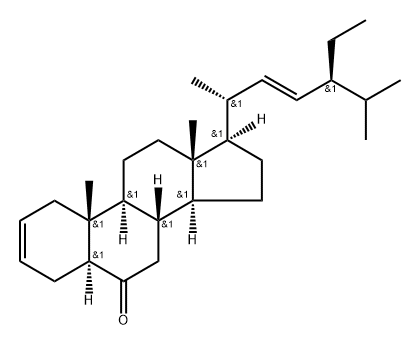
74174-45-1
1 suppliers
inquiry

82373-95-3
46 suppliers
inquiry