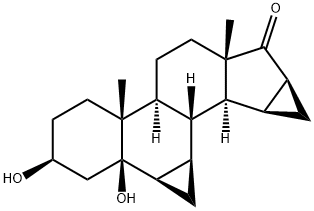
3b,5-Dihydroxy-6b,7b:15b,16b-dimethylene-5b-androstan-17-one synthesis
- Product Name:3b,5-Dihydroxy-6b,7b:15b,16b-dimethylene-5b-androstan-17-one
- CAS Number:82543-16-6
- Molecular formula:C21H30O3
- Molecular Weight:330.46
![(3b,5b,15a,16a)-15,16-Dihydro-3,5-dihydroxy-3'H-cycloprop[15,16]androsta-6,15-dien-17-one](/CAS/GIF/82543-15-5.gif)
82543-15-5

74-95-3

82543-16-6
Zinc powder (135.00 g, 2.065 mol) and copper (I) bromide (5.00 g, 0.035 mol) were suspended in 1,2-dimethoxyethane (1820 mL) under nitrogen protection. The mixture was heated to 75 °C and stirred for 20 min. Next, acetic acid (4.5 mL, 0.078 mol) and isopropanol (9.5 mL, 0.124 mol) were added to the system and stirred at 72 °C. A solution of dibromomethane (334 g, 1.921 mol) in 1,2-dimethoxyethane (114 mL) was added slowly dropwise, with the rate of acceleration of the drop being controlled to maintain the reaction temperature at about 75 °C. The reaction mixture was stirred for 1 hour until TLC analysis showed complete conversion of the feedstock. After addition of ethyl acetate (2500 mL), the system was cooled to 0-5 °C. A 5% aqueous acetic acid solution (2500mL) was added slowly and stirring was continued for 30 minutes. The solid was collected by filtration and the liquid phase was separated. The aqueous layer was extracted twice each with ethyl acetate (1250 mL, 500 mL) and the combined organic phases were washed twice with distilled water (2 x 2500 mL) and separated. The organic solvent was removed under reduced pressure at 45 °C and the residue was dissolved in diisopropyl ether (560 mL), stirred, filtered and washed twice with diisopropyl ether. The resulting (2S,4aR,4bS,6aS,7aS,8aS,8bS,8cR,8dR,9aR,9bR)-2.9b-dihydroxy-4a,6a-dimethyloctadecyl-7H-cyclopropa[4,5]cyclo-penta[1,2-a]cyclopropa[l]phenanthracenecarbenecarbenone 7-ketone was dried in vacuo at 45 °C to give the product 74.50 g (0.225 mol, 71% yield). The product was structurally confirmed by 1H-NMR and 13C-NMR, and HPLC-MS (ESI) analysis showed [M + Na]+ = 353 and [2M + Na]+ = 683.
![(3b,5b,15a,16a)-15,16-Dihydro-3,5-dihydroxy-3'H-cycloprop[15,16]androsta-6,15-dien-17-one](/CAS/GIF/82543-15-5.gif)
82543-15-5
109 suppliers
$120.50/50 mg

74-95-3
265 suppliers
$10.00/10g

82543-16-6
128 suppliers
$58.00/1g
Yield: 71%
Reaction Conditions:
with acetic acid;copper(I) bromide;zinc in 1,2-dimethoxyethane;isopropyl alcohol at 72 - 75; for 1 h;
Steps:
5 3β,5-Dihydroxy-6β,7β,15β,16β-dimethylen-5β-androst-17-one
EXAMPLE 5 3β,5-Dihydroxy-6β,7β,15β,16β-dimethylen-5β-androst-17-one Zinc (powder, 135.00 g, 2.065 mol) and copper (I) bromide (5.00 g, 0.035 mol) are suspended in 1,2-dimethoxyethane (1820 mL) under nitrogen atmosphere; the mixture is heated up to 75°C and stirred for 20 minutes. 3β,5-dihydroxy-15β,16β-methylen-5β-androst-6-en-17-one (100.00 g, 0.316 mol) is added and the mixture is stirred for 15 minutes. The system is then treated with acetic acid (4.5 mL, 0.078 mol) and isopropanol (9.5 mL, 0.124 mol) and stirred at 72°C. A solution of dibromomethane (334 g, 1.921 mol) in 1,2-dimethoxyethane (114 mL) is added dropwise at such a rate that the temperature is maintained around 75°C. The mixture is stirred for one hour until TLC analysis reveals that the conversion of the starting material is complete. Ethyl acetate (2500 mL) is added and the system is cooled to 0-5°C. After 5% acetic acid in water (2500 L) is slowly added, stirring is maintained for 30 minutes. The solid is filtered and the liquid phases are separated. The aqueous layer is extracted twice with ethyl acetate (1250 mL, 500 mL) and the combined organic phases are washed twice with water (2 x 2500 mL) and separated. After removing the organic solvent at 45°C under reduced pressure the residue is taken up in diisopropyl ether (560 mL), stirred, filtered and washed twice with diisopropyl ether. The title compound is dried at 45°C in vacuo (74.50 g, 0.225 mol, 71 %). 1H-NMR {200 MHz, CDCl3, δ (ppm)}: 0.86 (s, 3H, CH3-18); 0.6-2.3 (21 H); 0.95 (s, 3H, CH3-19); 3.00 (bs, 2H, 2(OH)); 4.06 (m, 1H, H-3). 13C-NMR {200 MHz, CDCl3, δ (ppm)}: 11.7 (CH2); 13.9 (CH); 17.6 (CH2); 19.0 (CH3); 20.1 (CH3); 21.1 (CH2); 22.3 (CH); 25.0 (CH); 25.9 (CH); 26.7 (CH2); 27.6 (CH2); 33.3 (CH); 34.9 (CH2); 40.5 (C); 43.0 (C); 42.9 (CH2); 45.4 (CH); 51.9 (CH); 66.9 (CH); 74.5 (C); 216.3 (C). HPLC-MS (ESI): [M+Na]+ = 353; [2M+Na]+ = 683
References:
Newchem S.p.A. EP2019114, 2009, A1 Location in patent:Page/Page column 11

75-11-6
349 suppliers
$10.00/5g
![(3b,5b,15a,16a)-15,16-Dihydro-3,5-dihydroxy-3'H-cycloprop[15,16]androsta-6,15-dien-17-one](/CAS/GIF/82543-15-5.gif)
82543-15-5
109 suppliers
$120.50/50 mg

82543-16-6
128 suppliers
$58.00/1g
![15,16-Dihydro-3-hydroxy-3'H-cycloprop[15,16]androsta-5,15-dien-17-one](/CAS/GIF/67372-65-0.gif)
67372-65-0
10 suppliers
inquiry

82543-16-6
128 suppliers
$58.00/1g
![3H-Cycloprop[15,16]androsta-5,15-dien-17-one,15,16-dihydro-3,7-dihydroxy-,](/CAS/GIF/82543-07-5.gif)
82543-07-5
1 suppliers
inquiry

82543-16-6
128 suppliers
$58.00/1g
![3'H-Cycloprop[15,16]androsta-6,15-dien-17-one, 3-(2,2-dimethyl-1-oxopropoxy)-15,16-dihydro-5-hydroxy-, (3β,5β,15α,16α)-](/CAS/20211123/GIF/82543-14-4.gif)
82543-14-4
2 suppliers
inquiry

82543-16-6
128 suppliers
$58.00/1g