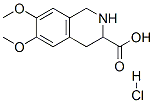
1,2,3,4-Tetrahydro-6,7-dimethoxy-3-isoquinolinecarboxylic acid hydrochloride synthesis
- Product Name:1,2,3,4-Tetrahydro-6,7-dimethoxy-3-isoquinolinecarboxylic acid hydrochloride
- CAS Number:82586-62-7
- Molecular formula:C12H16ClNO4
- Molecular Weight:273.71

358627-67-5
2 suppliers
inquiry

82586-62-7
134 suppliers
$27.00/1g
Yield: 90.9%
Reaction Conditions:
with hydrogenchloride in methanol;water at 60; for 2 h;
Steps:
9
To a solution of (S)-1,2,3,4-tetrahydro-6,7-dimethoxy-2-(tert-butyloxycarbonyl)-3-isoquinolinecarboxylic acid (example 3) (60 g, 0.17 mol) in methanol (300 mL) was added 3 N aqueous HCl (240 mL, 0.72 mol). The mixture was heated to 60° C. and stirred at 60° C. for 2 hrs. The clear reaction solution was evaporated under vacuum to 200 mL. The resulting white suspension was then cooled to 0-5° C. and stirred at 0-5° C., filtered, and rinsed with cold water (2×35 mL). The solid was dried under vacuum to give 42 g of (S)-1,2,3,4-tetrahydro-6,7-dimethoxy-3-isoquinolinecarboxylic acid hydrochloride (yield 90.9%, ee>98%). 1H NMR (DMSO-d6) δ: 3.06 (dd, J=16.7, 10.8 Hz, 1H, CHHCHCOOH), 3.22 (dd, J=16.7, 4.7 Hz, 1H, CHHCHCOOH), 3.72, 3.73 (s, 6 H, OCH3), 4.21 (br s, 2H, CH2N), 4.32 (dd, J=10.8, 4.7 Hz, 1H, CHCOOH), 6.85, 6.87 (s, 2H, PhH), 10.12 (br s, 2H, NHHCl).
References:
Brantford Chemicals Inc. US6642384, 2003, B1 Location in patent:Page/Page column 11

50-00-0
846 suppliers
$10.00/25g
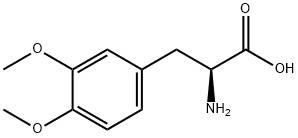
32161-30-1
160 suppliers
$28.00/1G

82586-62-7
134 suppliers
$27.00/1g
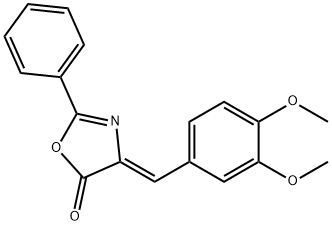
25349-37-5
2 suppliers
inquiry

82586-62-7
134 suppliers
$27.00/1g
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

82586-62-7
134 suppliers
$27.00/1g
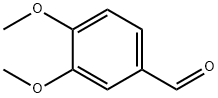
120-14-9
752 suppliers
$5.00/25g

82586-62-7
134 suppliers
$27.00/1g