
6-(2,4-Dioxo-1,4-dihydroquinazolin-3(2H)-yl)-hexanoic acid synthesis
- Product Name:6-(2,4-Dioxo-1,4-dihydroquinazolin-3(2H)-yl)-hexanoic acid
- CAS Number:82603-64-3
- Molecular formula:C14H16N2O4
- Molecular Weight:276.29
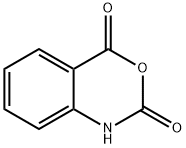
118-48-9
411 suppliers
$5.00/5G
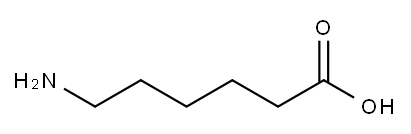
60-32-2
588 suppliers
$5.00/10g

82603-64-3
11 suppliers
$158.00/500mg
Yield:82603-64-3 60%
Reaction Conditions:
Stage #1: isatoic anhydride;6-aminohexanoic acidwith triethylamine in lithium hydroxide monohydrate at 30 - 40; for 2 h;
Stage #2: with formic acid in lithium hydroxide monohydrate; for 7 h;Reflux;
Steps:
3.2.1. General Procedure (A) for the Synthesis of 2a-2c
General procedure: Triethylamine (1.0 equiv.) was added to a solution of a-c (1.0 equiv.) in water (2.6 mL)followed by a portion wise addition of isatoic anhydride 1 (1.1 equiv.). The reaction mixturewas stirred for 2 h at 30-40 °C, cooled to room temperature and evaporated in vacuum toform an oil residue. This material was refluxed for 7 h in formic acid (3.6mL), cooled toroom temperature and evaporated. The solid was resuspended in water, extracted withDCM (3 25 mL), dried over anhydrous Na2SO4, filtered, and concentrated. The desiredcompounds 2a-2c were confirmed by analytical RP-HPLC (Nucleodur, C8 reversed-phasecolumn: 100 2 mm, 4 M, 80 ?, flow rate = 1 mL/min) and used without any furtherpurification for the next step [74].
References:
Gazzillo, Erica;Terracciano, Stefania;Ruggiero, Dafne;Potenza, Marianna;Chini, Maria Giovanna;Lauro, Gianluigi;Fischer, Katrin;Hofstetter, Robert Klaus;Giordano, Assunta;Werz, Oliver;Bruno, Ines;Bifulco, Giuseppe [Molecules,2022,vol. 27,# 12,art. no. 3866]

118-48-9
411 suppliers
$5.00/5G
79-22-1
2 suppliers
$38.00/1000g

60-32-2
588 suppliers
$5.00/10g

82603-64-3
11 suppliers
$158.00/500mg