
2-(3-HYDROXY-PHENYL)-1,3-DIOXO-2,3-DIHYDRO-1H-ISOINDOLE-5-CARBOXYLIC ACID synthesis
- Product Name:2-(3-HYDROXY-PHENYL)-1,3-DIOXO-2,3-DIHYDRO-1H-ISOINDOLE-5-CARBOXYLIC ACID
- CAS Number:82811-05-0
- Molecular formula:C15H9NO5
- Molecular Weight:283.24
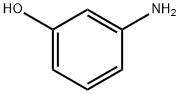
591-27-5
535 suppliers
$11.00/10g
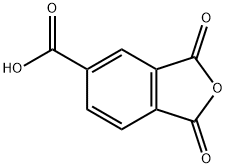
552-30-7
397 suppliers
$16.00/25g

82811-05-0
20 suppliers
$28.60/10MG
Yield:82811-05-0 95%
Reaction Conditions:
Stage #1: m-Hydroxyaniline;trimellitic Anhydride in 1-methyl-pyrrolidin-2-one at 20; for 6 h;Inert atmosphere;
Stage #2: in 1-methyl-pyrrolidin-2-one;toluene at 120; for 10 h;Dean-Stark;
Steps:
Synthesis of aromatic hydroxy acid
General procedure: N-(4-hydroxyphenyl)-4’-trimellitimide (IIIa). The stable aromatic hydroxy acids (IIIa-IIId) were prepared by the nucleophilic substitution reaction of trimellitic anhydride with aromatic amino phenol/amino naphthol [13-19]. The typical procedure adopted for the synthesis of the compound IIIa was as follows: in the first step, trimellitic anhydride (I) (0.02 mol) was added slowly under nitrogen atmosphere into the solution of 4-aminophenol (IIa) (0.04 mol) in polar aprotic solvent NMP (25 mL), and was stirred at room temperature for 6 h to form amic acid intermediate. In the second step, toluene (15 mL) was added into the amic acid intermediate and the resulting mixture was refluxed at 120 °C for 10 h to afford the cyclic diiimide IIIa. The water formed during this conversion was removed azeotropically using Dean-Stark trap (Scheme 1). After removing the water, the temperature of the reaction mixture was raised to distilled off the residual toluene. The reaction mixture was cooled, poured into water and the precipitated hydroxy acid compound IIIa was filtered, washed with excess of dil. HCl to remove the unreached 4-aminophenol and washed with excess of water, finally dried in vacuum oven at 80 °C
References:
Thiruvasagam [Bulletin of the Chemical Society of Ethiopia,2018,vol. 32,# 3,p. 523 - 530]