
3,5 - dibroMo - 6 - oxo - 6H - anthra<1,9 - cd>isoxazole synthesis
- Product Name:3,5 - dibroMo - 6 - oxo - 6H - anthra<1,9 - cd>isoxazole
- CAS Number:82840-40-2
- Molecular formula:C14H5Br2NO2
- Molecular Weight:379
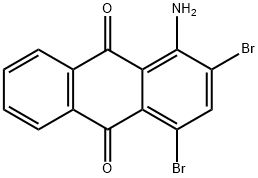
81-49-2
109 suppliers
$12.00/5g

82840-40-2
13 suppliers
inquiry
Yield:82840-40-2 76%
Reaction Conditions:
Stage #1: 1-amino-2,4-dibromoanthracene-9,10-dionewith sulfuric acid;NaNO2 in water monomer at 30 - 55; for 17 h;
Stage #2: with Caswell No. 744A in water monomer; for 16 h;
Stage #3: in toluene at 70; for 12 h;
Steps:
3,5-dibromo-6H-anthra[l,9-cd]isoxazol-6-one (1) - Sodium nitrite (993 mg, 14.4 mmol) was added with stirring to cone. H2SO4 (25 mL) at 30-40°C over 10 min then stirred for an additional 30 min. Next l-amino-2,4-dibromoanthraquinone (5.0 g, 13.1 mmol) was added over 15 min and the mixture was stirred overnight (16h) at 50-55°C. The heated solution was poured directly over ice and the resulting yellow precipitate was filtered, washed with cold water, and a 1 : 1 mixture of ethanol-ether. The moist anthraquinonediazonium hydrogensulfate was added to a solution of NaN3 (1.37 g, 21.0 mmol) in water (25 mL) and stirred overnight (16h). The light orange solid was filtered off and washed with water followed by a 9: 1 mixture of acetone-water. The moist azide was suspended in toluene (40 mL) and heated to 70°C with stirring. Water and acetone were slowly distilled (using a Dean-Stark apparatus) over a 12 h period. The yellow-orange crystals were filtered and washed with methanol to give 3.78 g (76%) of 3,5-dibromo-6H-anthra[l,9-cd]isoxazol-6-one. 1H NMR (500 MHz, CDC13) δ 8.44 (d, J = 7.8 Hz, 1H), 8.07 (d, J = 7.4 Hz, 1H), 7.97 (s, 1H), 7.80 (t, J = 7.0 Hz, 1H), 7.71 (t, J = 7.4 Hz, 1H). Reference: Sutter, P; Weis, CD. J. Heterocyclic Chem. 1982, 19, 997-1011.
References:
WO2012/119079,2012,A1 Location in patent:Page/Page column 40
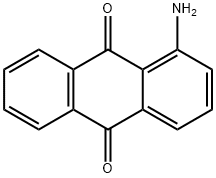
82-45-1
291 suppliers
$9.00/25g

82840-40-2
13 suppliers
inquiry
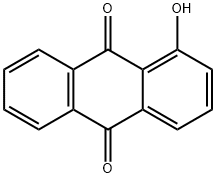
129-43-1
131 suppliers
$51.00/5g

82840-40-2
13 suppliers
inquiry
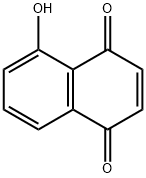
481-39-0
230 suppliers
$30.00/500mg

82840-40-2
13 suppliers
inquiry

130-15-4
397 suppliers
$16.00/1g

82840-40-2
13 suppliers
inquiry