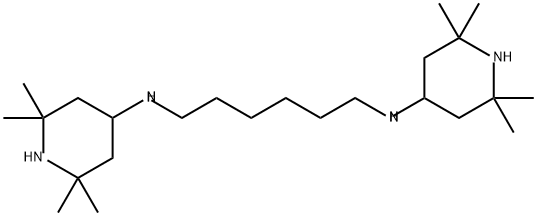
1,3,5-Triazine-2,4-diamine, N2,N2'-1,6-hexanediylbis[N4-butyl-6-chloro-N2,N4-bis(2,2,6,6-tetramethyl-4-piperidinyl)- synthesis
- Product Name:1,3,5-Triazine-2,4-diamine, N2,N2'-1,6-hexanediylbis[N4-butyl-6-chloro-N2,N4-bis(2,2,6,6-tetramethyl-4-piperidinyl)-
- CAS Number:83420-16-0
- Molecular formula:C56H102Cl2N14
- Molecular Weight:1042.41
Yield:83420-16-0 91 %
Reaction Conditions:
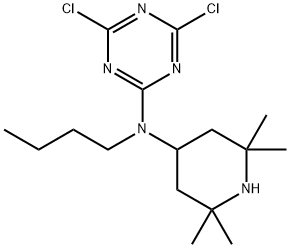
Stage #1: N,N'-bis(2,2,6,6-tetramethyl-4-piperidyl)-1,6-diaminohexane;N-butyl-4,6-dichloro-N-(2,2,6,6-tetramethylpiperidin-4-yl)-1,3,5-triazin-2-amine in m-xylene at 60;
Stage #2: with sodium hydroxide in water;m-xylene;
Steps:
1.2 Synthesis of the second intermediate V:
Take 17.4g (44.0mmol) hexamethylenediamine piperidine IV and 31.7g (88.0mmol)The first intermediate III is added to a four-neck round bottom flask of 500 mL, and a thermometer sleeve is assembled,Adding 200mL m-xylene in the reaction vessel is the first solvent to dissolve the raw material,Maintain the reaction system at 60°C (you can also choose any value between 60-75°C, such as 61, 62, 63, 64, 65, 67, 69, 71, 73, 75°C), while stirring continuously, react for 4h ( It can also be any time in 4-6h, such as 4.1, 4.5, 5, 5.5, 6h);Slowly add 17.6 mL of NaOH aqueous solution with a concentration of 20 wt %,After the dropwise addition was completed, the reaction was continued for 4h. Use TLC to indicate the end point, stop the reaction,The reaction solution was moved to a separatory funnel for phase separation, the water layer was separated, washed with a 10 wt% NaCl aqueous solution, and the resulting organic phase was placed in a refrigerator to freeze overnight, filtered,Dried to get white powder, i.e. the second intermediate V is 41g, as shown in Figure 2,The yield is 91%, and the purity is 99.6%.
References:
CN115710252,2023,A Location in patent:Paragraph 0037; 0042-0044