
(5Z)-5-(4-chlorobenzylidene)-2-(morpholin-4-yl)-1,3-thiazol-4(5H)-one synthesis
- Product Name:(5Z)-5-(4-chlorobenzylidene)-2-(morpholin-4-yl)-1,3-thiazol-4(5H)-one
- CAS Number:83539-49-5
- Molecular formula:C14H13ClN2O2S
- Molecular Weight:308.78

110-91-8
678 suppliers
$9.00/1g

141-84-4
251 suppliers
$8.00/5g
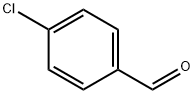
104-88-1
614 suppliers
$5.00/25g

83539-49-5
4 suppliers
inquiry
Yield:83539-49-5 89%
Reaction Conditions:
with silica-based substituted pyridine catalyst 7 in ethanol;water at 20; for 1.81667 h;diastereoselective reaction;Knoevenagel condensation;
Steps:
General experimental procedure for the synthesis of 2-amino-5-alkylidenethiazol-4-ones:
General procedure: A mixture of rhodanine (0.25 mol/L), amine (0.25 mol/L), and ketone (0.25 mol/L) and 40 mg of silica based substituted pyridine catalyst in aqueous ethanol [(2+2) ml] were stirred in room temperature until the reaction is completed. The completion of the reaction was indicated by the disappearance of the starting material in thin layer chromatography. The products precipitated out once their formation started. After completion of the reaction the crude product was filtered and the residue was taken in DCM. It was again filtered to separate the product as filtrate from the catalyst which is separated as residue. It was further purified either by recrystallization from EtOAc/DCM (equal volume).
References:
Mukhopadhyay, Chhanda;Ray, Suman [Tetrahedron Letters,2011,vol. 52,# 48,p. 6431 - 6438] Location in patent:experimental part